Association of Hippocampal Subfield Volumes with Amyloid-Beta Deposition in Alzheimer's Disease
- PMID: 35329851
- PMCID: PMC8955328
- DOI: 10.3390/jcm11061526
Association of Hippocampal Subfield Volumes with Amyloid-Beta Deposition in Alzheimer's Disease
Abstract
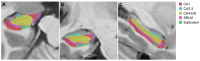
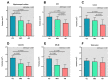
We investigated the relationship between hippocampal subfield volumes and cortical amyloid-beta (Aβ) deposition in Alzheimer’s disease (AD). Fifty participants (11 cognitively unimpaired [CU], 10 with mild cognitive impairment [MCI], and 29 with AD) who underwent 18F-florbetaben positron emission tomography, magnetic resonance imaging, and neuropsychological tests were enrolled. The hippocampal subfield volumes were obtained using an automated brain volumetry system with the Winterburn atlas and were compared among the diagnostic groups, and the correlations with the Aβ deposition and AD risk factors were determined. Patients with MCI and AD showed decreased volume in the stratum radiatum/lacunosum/moleculare (SRLM) of the cornu ammonis (CA)1 and CA4-dentate gyrus (DG) compared with the CU. Decreased SRLM and CA4-DG volumes were associated with an increased Aβ deposition in the global cortex (R = −0.459, p = 0.001; R = −0.393, p = 0.005, respectively). The SRLM and CA4-DG volumes aided in the distinction of AD from CU (areas under the receiver operating characteristic [AUROC] curve = 0.994 and 0.981, respectively, p < 0.001), and Aβ+ from Aβ− individuals (AUROC curve = 0.949 and 0.958, respectively, p < 0.001). Hippocampal subfield volumes demonstrated potential as imaging biomarkers in the diagnosis and detection of AD and Aβ deposition, respectively.
Keywords: Alzheimer’s disease; amyloid-beta; biomarker; hippocampal subfields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jack C.R., Jr., Lowe V.J., Senjem M.L., Weigand S.D., Kemp B.J., Shiung M.M., Knopman D.S., Boeve B.F., Klunk W.E., Mathis C.A. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. - DOI - PMC - PubMed
-
- Jack C.R., Wiste H.J., Vemuri P., Weigand S.D., Senjem M.L., Zeng G.A., Bernstein M.A., Gunter J.L., Pankratz V.S., Aisen P.S., et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. - DOI - PMC - PubMed
-
- Cardenas V.A., Chao L.L., Studholme C., Yaffe K., Miller B.L., Madison C., Buckley S.T., Mungas D., Schuff N., Weiner M.W. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol. Aging. 2011;32:572–580. doi: 10.1016/j.neurobiolaging.2009.04.011. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

