Long-Term Efficacy and Safety of Rifaximin in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study
- PMID: 35329897
- PMCID: PMC8948903
- DOI: 10.3390/jcm11061571
Long-Term Efficacy and Safety of Rifaximin in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study
Abstract
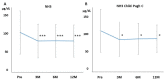
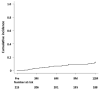
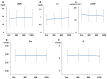
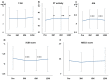
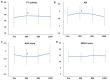
Background: Rifaximin is commonly used for hepatic encephalopathy (HE). However, the effects of long-term treatment for Japanese people are limited. Therefore, this study aimed to investigate the effects and safety of long-term treatment with rifaximin on HE. Methods: A total of 215 patients with cirrhosis administered with rifaximin developed overt or covert HE, which was diagnosed by an attending physician for >12 months. Laboratory data were extracted at pretreatment and 3, 6, and 12 months after rifaximin administration. The long-term effect of rifaximin was evaluated, and the incidence of overt HE during 12 months and adverse events was extracted. Results: Ammonia levels were significantly improved after 3 months of rifaximin administration and were continued until 12 months. There were no serious adverse events after rifaximin administration. The number of overt HE incidents was 9, 14, and 27 patients within 3, 6, and 12 months, respectively. Liver enzymes, renal function, and electrolytes did not change after rifaximin administration. Prothrombin activity is a significant risk factor for the occurrence of overt HE. The serum albumin, prothrombin activity, and albumin−bilirubin (ALBI) scores were statistically improved after 3 and 6 months of rifaximin administration. Moreover, the same results were obtained in patients with Child−Pugh C. Conclusions: The long-term rifaximin treatment was effective and safe for patients with HE, including Child−Pugh C.
Keywords: Japanese; cirrhosis; hepatic encephalopathy; long-term; rifaximin.
Conflict of interest statement
Satoshi Mochida, Shuji Terai, Yoshiyuki Ueno, Kazuhiko Koike, and Hitoshi Yoshiji received lecture fees provided by ASKA Pharmaceutical Co., Ltd. Satoshi Mochida, Shuji Terai, Yoshiyuki Ueno, and Kazuhiko Koike received research funding from ASKA Pharmaceutical Co., Ltd. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- American Association for the Study of Liver Diseases. European Association for the Study of the Liver Hepatic enceph-alopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. - DOI - PubMed
-
- Greinert R., Zipprich A., Simón-Talero M., Stangl F., Ludwig C., Wienke A., Praktiknjo M., Höhne K., Trebicka J., Genescà J., et al. Covert hepatic encephalopathy and spontaneous portosystemic shunts increase the risk of developing overt hepatic encephalopathy. Liver Int. 2020;40:3093–3102. doi: 10.1111/liv.14660. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

