The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor
- PMID: 35329960
- PMCID: PMC8954265
- DOI: 10.3390/jcm11061634
The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor
Abstract
Background: Injectable cardiac monitors (ICMs) are leadless subcutaneous devices for long-term monitoring of arrhythmias. The BIOTRONIK BIOMONITOR III is a novel ICM with a miniaturized profile, long sensing vector, and simplified implantation technique.
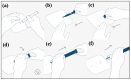
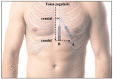
Methods: R-wave amplitude was recorded immediately after implantation, the day after implantation, and after 3 months. Follow-up was scheduled after 3 months or after an event. All data from the ICM were retrieved. The anatomical position of the ICM was determined post-implantation and after 3 months. A patient questionnaire was conducted after 3 months.
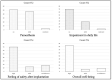
Results: In 36 patients (mean age 67 ± 13 years; 40% male) an ICM was inserted. Six patients were not included in the final analysis. The median time from skin cut to wound closure was 6 [IQR 5-7] minutes. Mean R-wave amplitude increased over time (0.73 ± 32 mV vs. 0.78 ± 0.38 mV vs. 0.81 ± 0.39 mV; p = ns). Three months after implantation, the ICM was in an anatomically stable position. In 14 (47%) patients, true episodes were detected. False arrhythmia alerts were detected in 13 (43%) patients. The total number of false detections was low, and the patient satisfaction rate was high.
Conclusion: Implantation of the novel BIOMONITOR III is fast and uncomplicated; its sensing characteristics are excellent and improve over time, and patient satisfaction is high.
Keywords: BIOMONITOR III; R-wave sensing; implantable cardiac monitor; implantable loop recorder; signal quality.
Conflict of interest statement
N.R. has received honoraria outside the content of this study. The authors declare no conflict of interest. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Implantation of a novel insertable cardiac monitor: preliminary multicenter experience in Europe.J Interv Card Electrophysiol. 2024 Dec;67(9):2117-2125. doi: 10.1007/s10840-024-01821-y. Epub 2024 May 16. J Interv Card Electrophysiol. 2024. PMID: 38755520 Free PMC article. Review.
-
The BioMonitor 2 insertable cardiac monitor: Clinical experience with a novel implantable cardiac monitor.J Electrocardiol. 2018 Sep-Oct;51(5):751-755. doi: 10.1016/j.jelectrocard.2018.05.017. Epub 2018 May 29. J Electrocardiol. 2018. PMID: 30177307
-
Miniaturized implantable cardiac monitor with a long sensing vector (BIOMONITOR III): Insertion procedure assessment, sensing performance, and home monitoring transmission success.J Electrocardiol. 2020 May-Jun;60:118-125. doi: 10.1016/j.jelectrocard.2020.04.004. Epub 2020 Apr 11. J Electrocardiol. 2020. PMID: 32361086
-
New-generation miniaturized insertable cardiac monitor with a long sensing vector: Insertion procedure, sensing performance, and home monitoring transmission success in a real-world population.Heart Rhythm O2. 2022 Jan 30;3(2):152-159. doi: 10.1016/j.hroo.2022.01.010. eCollection 2022 Apr. Heart Rhythm O2. 2022. PMID: 35496450 Free PMC article.
-
Insertable cardiac monitors: current indications and devices.Expert Rev Med Devices. 2019 Jan;16(1):45-55. doi: 10.1080/17434440.2018.1557046. Epub 2018 Dec 11. Expert Rev Med Devices. 2019. PMID: 30522350 Review.
Cited by
-
ICM Implantation and Remote Follow-Up Management by Trained Nurses in Italian Hospitals: Current Practice and Nurse Feedback.J Cardiovasc Electrophysiol. 2025 Apr;36(4):741-759. doi: 10.1111/jce.16582. Epub 2025 Jan 29. J Cardiovasc Electrophysiol. 2025. PMID: 39887599 Free PMC article.
-
Changes in R-wave amplitude at implantation are associated with gender and orientation of insertable cardiac monitor: observations from the confirm Rx™ body posture and physical activity study.BMC Cardiovasc Disord. 2022 Oct 8;22(1):439. doi: 10.1186/s12872-022-02752-0. BMC Cardiovasc Disord. 2022. PMID: 36209063 Free PMC article. Clinical Trial.
-
Implantation of a novel insertable cardiac monitor: preliminary multicenter experience in Europe.J Interv Card Electrophysiol. 2024 Dec;67(9):2117-2125. doi: 10.1007/s10840-024-01821-y. Epub 2024 May 16. J Interv Card Electrophysiol. 2024. PMID: 38755520 Free PMC article. Review.
-
Implantable Loop Recorder with Long Sensing Vector: Safety, Acceptability, and Sensing Performance in Pediatric Patients.Pediatr Cardiol. 2023 Jun;44(5):1068-1075. doi: 10.1007/s00246-022-03082-w. Epub 2022 Dec 28. Pediatr Cardiol. 2023. PMID: 36576525 Free PMC article.
References
-
- Kapa S., Epstein A.E., Callans D.J., Garcia F.C., Lin D., Bala R., Riley M.P., Hutchinson M., Gerstenfeld E.P., Tzou W., et al. Assessing Arrhythmia Burden After Catheter Ablation of Atrial Fibrillation Using an Implantable Loop Recorder: The ABACUS Study. J. Cardiovasc. Electrophysiol. 2013;24:875–881. doi: 10.1111/jce.12141. - DOI - PubMed
-
- Pecha S., Aydin M.A., Ahmadzade T., Hartel F., Hoffmann B., Steven D., Willems S., Reichenspurner H., Wagner F.M. Implantable loop recorder monitoring after concomitant surgical ablation for atrial fibrillation (AF): Insights from more than 200 continuously monitored patients. Heart Vessel. 2016;31:1347–1353. doi: 10.1007/s00380-015-0735-4. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

