Comparison of the Drug-Induced Efficacies between Omidenepag Isopropyl, an EP2 Agonist and PGF2α toward TGF-β2-Modulated Human Trabecular Meshwork (HTM) Cells
- PMID: 35329980
- PMCID: PMC8954773
- DOI: 10.3390/jcm11061652
Comparison of the Drug-Induced Efficacies between Omidenepag Isopropyl, an EP2 Agonist and PGF2α toward TGF-β2-Modulated Human Trabecular Meshwork (HTM) Cells
Abstract
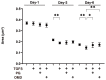
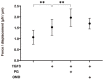
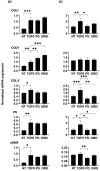
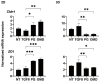
To compare the drug-induced efficacies between omidenepag (OMD), an EP2 agonist, and prostaglandin F2α (PGF2α) on glaucomatous trabecular meshwork (TM) cells, two- and three-dimensional (2D and 3D) cultures of TGF-β2-modulated human trabecular meshwork (HTM) cells were used. The following analyses were performed: (1) transendothelial electrical resistance (TEER) and FITC-dextran permeability measurements (2D), (2) the size and stiffness of the 3D spheroids, and (3) the expression (both 2D and 3D) by several extracellular matrix (ECM) molecules including collagen (COL) 1, 4 and 6, and fibronectin (FN), and α smooth muscle actin (αSMA), tight junction (TJ)-related molecules, claudin11 (Cldn11) and ZO1, the tissue inhibitor of metalloproteinase (TIMP) 1-4, matrix metalloproteinase (MMP) 2, 9 and 14, connective tissue growth factor (CTGF), and several endoplasmic reticulum (ER) stress-related factors. TGF-β2 significantly increased the TEER values and decreased FITC-dextran permeability, respectively, in the 2D HTM monolayers, and induced the formation of downsized and stiffer 3D HTM spheroids. TGF-β2-induced changes in TEER levels and FITC-dextran permeability were remarkably inhibited by PGF2α. PGF2α induced increases in the sizes and stiffness of the TGF-β2-treated 3D spheroids, but OMD enhanced only spheroid size. Upon exposure to TGF-β2, the expression of most of the molecules that were evaluated were significantly up-regulated, except some of ER stress-related factors were down-regulated. TJ-related molecules or ER stress-related factors were significantly up-regulated (2D) or down-regulated (3D), and down-regulated (2D) by PGF2α and OMD, while both drugs altered the expression of some of the other genes in the 3D spheroids in a different manner. The findings presented herein suggest that PGF2α and OMD differently modulate the permeability of the TGFβ2-modulated 2D monolayers and the physical properties of the 3D HTM spheroids.
Keywords: 3D spheroid cultures; PGF2α; TGFβ2; human trabecular meshwork; omidenepag; omidenepag isopropyl.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Screening of the Drug-Induced Effects of Prostaglandin EP2 and FP Agonists on 3D Cultures of Dexamethasone-Treated Human Trabecular Meshwork Cells.Biomedicines. 2021 Jul 31;9(8):930. doi: 10.3390/biomedicines9080930. Biomedicines. 2021. PMID: 34440134 Free PMC article.
-
The EP2 agonist, omidenepag, alters the physical stiffness of 3D spheroids prepared from human corneal stroma fibroblasts differently depending on the osmotic pressure.FASEB J. 2022 Jan;36(1):e22067. doi: 10.1096/fj.202101263R. FASEB J. 2022. PMID: 34914140 Clinical Trial.
-
All-trans Retinoic Acids Synergistically and Beneficially Affect In Vitro Glaucomatous Trabecular Meshwork (TM) Models Using 2D and 3D Cell Cultures of Human TM Cells.Int J Mol Sci. 2022 Aug 31;23(17):9912. doi: 10.3390/ijms23179912. Int J Mol Sci. 2022. PMID: 36077314 Free PMC article.
-
The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma.J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2-3):154-62. doi: 10.1089/jop.2013.0220. Epub 2014 Feb 11. J Ocul Pharmacol Ther. 2014. PMID: 24517218 Free PMC article. Review.
-
Modulation of extracellular matrix turnover in the trabecular meshwork.Exp Eye Res. 2009 Apr;88(4):683-8. doi: 10.1016/j.exer.2009.01.005. Exp Eye Res. 2009. PMID: 19385040 Review.
Cited by
-
Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review.Cells. 2024 Sep 14;13(18):1549. doi: 10.3390/cells13181549. Cells. 2024. PMID: 39329734 Free PMC article. Review.
-
Effect of antibody-mediated connective tissue growth factor neutralization on lung edema in ventilator-induced lung injury in rats.Mol Med. 2024 May 22;30(1):68. doi: 10.1186/s10020-024-00829-4. Mol Med. 2024. PMID: 38778274 Free PMC article.
-
Increasing the tumour targeting of antitumour drugs through anlotinib-mediated modulation of the extracellular matrix and the RhoA/ROCK signalling pathway.J Pharm Anal. 2024 Aug;14(8):100984. doi: 10.1016/j.jpha.2024.100984. Epub 2024 Apr 25. J Pharm Anal. 2024. PMID: 39258171 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

