Hypersensitivity Myocarditis after COVID-19 mRNA Vaccination
- PMID: 35329986
- PMCID: PMC8949349
- DOI: 10.3390/jcm11061660
Hypersensitivity Myocarditis after COVID-19 mRNA Vaccination
Abstract
Background: Myocarditis, even in a severe and lethal form, may occur after COVID-19 mRNA (BNT162b2) vaccination. However, its pathway, morphomolecular characterization and treatment are still unknown.
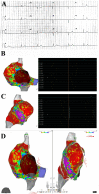
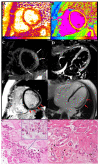
Methods: Routine hematochemical screening, ECG, Holter monitoring, 2D echocardiogram cardiac magnetic resonance (CMR) and invasive cardiac studies (cardiac catheterization, selective coronary angiography, left ventriculography and left ventricular endomyocardial biopsy) are reported from three patients (39F-pt1, 78M-pt2, 52M-pt3) with severe compromise of conduction tissue (junctional rhythm and syncope, pt1) or cardiac function compromise (LVEF ≤ 35%, pt2 and pt3) after COVID-19 mRNA (BNT162b2).
Results: Hematochemical data and coronary angiography were normal in the patients studied. Histology showed in all three patients extensive myocardial infiltration of degranulated eosinophils and elevation of serum cationic protein directly responsible for cardiomyocyte damage. These findings demonstrate myocarditis hypersensitivity to some component of the vaccine (spike protein?) acting as a hapten to some macromolecules of cardiomyocytes. Steroid administration (prednisone, 1 mg/kg die for 3 days, followed by 0.33 mg/kg for 4 weeks) was followed by complete recovery of cardiac contractility in pt2 and pt3.
Conclusions: Eosinophilic myocarditis is a possible adverse reaction to the mRNA COVID-19 vaccine. Its pathway is mediated by release of cationic protein and responds to short courses of steroid administration.
Keywords: COVID-19 mRNA vaccination; eosinophilic myocarditis; myocarditis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Aretz H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J., Fenoglio J.J., Olsen E.G., Schoen F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987;1:3–14. - PubMed
-
- Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

