Heparanase Inhibition Prevents Liver Steatosis in E0 Mice
- PMID: 35329997
- PMCID: PMC8954723
- DOI: 10.3390/jcm11061672
Heparanase Inhibition Prevents Liver Steatosis in E0 Mice
Abstract
Background: Non-alcoholic fatty liver disease affects up to 30% of adults in the USA, and is associated with a higher incidence of chronic liver morbidity and mortality. Several molecular pathways are involved in the pathology of liver steatosis, including lipid uptake, lipogenesis, lipolysis, and beta-oxidation. The enzyme heparanase has been implicated in liver steatosis. Herein, we investigated the effect of heparanase inhibition on liver steatosis in E0 mice.
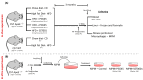
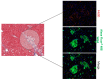
Methods: In vivo experiments: Male wild-type mice fed with either chow diet (n = 4) or high-fat diet (n = 6), and male E0 mice fed with chow diet (n = 8) or high-fat diet (n = 33) were included. Mice on a high-fat diet were treated for 12 weeks with PG545 at low dose (6.4 mg/kg/week, ip, n = 6) or high dose (13.3 mg/kg/week, ip, n = 7), SST0001 (1.2 mg/mouse/day, ip, n = 6), or normal saline (control, n = 14). Animals were sacrificed two days after inducing peritonitis. Serum was analyzed for biochemical parameters. Mouse peritoneal macrophages (MPMs) were harvested and analyzed for lipid content. Livers were harvested for histopathological analysis of steatosis, lipid content, and the expression of steatosis-related factors at the mRNA level. In vitro experiments: MPMs were isolated from untreated E0 mice aged 8-10 weeks and were cultured and treated with either PG545 or SST0001, both at 50 µg/mL for 24 h, followed by assessment of mRNA expression of steatosis related factors.
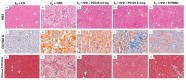
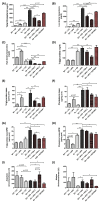
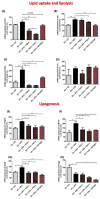
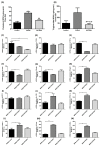
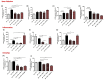
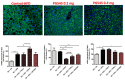
Results: Heparanase inhibition significantly attenuated the development of liver steatosis, as was evident by liver histology and lipid content. Serum analysis indicated lowering of cholesterol and triglycerides levels in mice treated with heparanase inhibitors. In liver tissue, assessment of mRNA expression of key factors in lipid uptake, lipolysis, lipogenesis, and beta-oxidation exhibited significant downregulation following PG545 treatment and to a lesser extent when SST0001 was applied. However, in vitro treatment of MPMs with PG545, but not SST0001, resulted in increased lipid content in these cells, which is opposed to their effect on MPMs of treated mice. This may indicate distinct regulatory pathways in the system or isolated macrophages following heparanase inhibition.
Conclusion: Heparanase inhibition significantly attenuates the development of liver steatosis by decreasing tissue lipid content and by affecting the mRNA expression of key lipid metabolism regulators.
Keywords: E0 mice; PG545; SST0001; heparanase; liver steatosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397.e310. doi: 10.1053/j.gastro.2015.04.043. - DOI - PMC - PubMed
-
- Jojima T., Tomotsune T., Iijima T., Akimoto K., Suzuki K., Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol. Metab. Syndr. 2016;8:45. doi: 10.1186/s13098-016-0169-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

