Does Repeated Dosing of Intravenous Ferric Carboxymaltose Alleviate Symptoms of Restless Legs Syndrome?
- PMID: 35329998
- PMCID: PMC8949271
- DOI: 10.3390/jcm11061673
Does Repeated Dosing of Intravenous Ferric Carboxymaltose Alleviate Symptoms of Restless Legs Syndrome?
Abstract
Background: Several studies have reported the efficacy of intravenous (IV) iron for patients with restless legs syndrome (RLS), but little is known about the efficacy or safety of repeated IV iron treatment. The aim of this study was to evaluate the effectiveness of repeated doses of IV ferric carboxymaltose (FCM) in treating RLS symptoms.
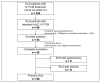
Methods: Patients who received FCM more than twice for RLS from April 2016 to January 2020 were retrospectively reviewed. Patients who had shown positive response to initial IV FCM re-visited the clinic when their symptoms returned, and received repeated IV FCM (1000 mg). Blood iron panels were measured before initial and repeated IV FCM. We defined 'responders' as patients with a greater than 40% decrease in International Restless Legs Study Group Severity Scale (IRLS) compared with pre-treatment levels.
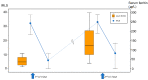
Results: A total of 42 patients, including 26 with primary RLS, 5 with gastrectomy, and 11 with anemia, completed the evaluation. Patients received IV FCM infusion 2-4 times. A total of 21 of 26 (80.8%) primary cases of RLS, 4 of 5 (80.0%) patients with a history of gastrectomy, and 9 of 11 (81.8%) patients with anemia responded to repeated FCM treatment. Serum ferritin levels of patients with primary RLS were higher before the second treatment than the baseline levels. There were no serious adverse events observed in the study.
Conclusions: Repeated IV FCM for recurring symptoms is an effective treatment for primary RLS and RLS associated with iron deficiency. Serum ferritin might not be a reliable factor to monitor the sustained effects of IV iron for RLS.
Keywords: RLS; ferric carboxymaltose; intravenous; iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Efficacy and safety of intravenous ferric carboxymaltose in the treatment of Restless Legs Syndrome: a systematic review and meta-analysis.Front Neurol. 2025 Jan 7;15:1503342. doi: 10.3389/fneur.2024.1503342. eCollection 2024. Front Neurol. 2025. PMID: 39839869 Free PMC article.
-
Patient characteristics predicting responses to intravenous ferric carboxymaltose treatment of restless legs syndrome.Sleep Med. 2020 Nov;75:81-87. doi: 10.1016/j.sleep.2020.02.027. Epub 2020 Mar 7. Sleep Med. 2020. PMID: 32853922
-
Clinical efficacy and safety of intravenous ferric carboxymaltose treatment for restless legs symptoms and low serum ferritin in children with autism spectrum disorder.Sleep Med. 2022 Dec;100:488-493. doi: 10.1016/j.sleep.2022.09.021. Epub 2022 Sep 30. Sleep Med. 2022. PMID: 36265207
-
Randomized, placebo-controlled trial of ferric carboxymaltose in restless legs syndrome patients with iron deficiency anemia.Sleep Med. 2021 Aug;84:179-186. doi: 10.1016/j.sleep.2021.05.036. Epub 2021 Jun 20. Sleep Med. 2021. PMID: 34157632 Clinical Trial.
-
Iron supplementation for restless legs syndrome - A systematic review and meta-analysis.Eur J Intern Med. 2019 May;63:34-41. doi: 10.1016/j.ejim.2019.02.009. Epub 2019 Feb 22. Eur J Intern Med. 2019. PMID: 30798983
Cited by
-
Efficacy and safety of intravenous ferric carboxymaltose in the treatment of Restless Legs Syndrome: a systematic review and meta-analysis.Front Neurol. 2025 Jan 7;15:1503342. doi: 10.3389/fneur.2024.1503342. eCollection 2024. Front Neurol. 2025. PMID: 39839869 Free PMC article.
-
Cerebral blood flow changes in maintenance hemodialysis patients with restless legs syndrome and their clinical significance:a cross-sectional case-control study.BMC Neurol. 2024 Apr 16;24(1):128. doi: 10.1186/s12883-024-03636-w. BMC Neurol. 2024. PMID: 38627680 Free PMC article.
References
Grants and funding
- 2021R1G1A1008471/National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT)
- OTC1190671/Samsung Medical Center Grant
- HR21C0885/Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea
LinkOut - more resources
Full Text Sources
Miscellaneous

