Efficacy of Pre-Procedural Mouthwashes against SARS-CoV-2: A Systematic Review of Randomized Controlled Trials
- PMID: 35330016
- PMCID: PMC8955331
- DOI: 10.3390/jcm11061692
Efficacy of Pre-Procedural Mouthwashes against SARS-CoV-2: A Systematic Review of Randomized Controlled Trials
Abstract
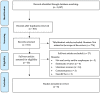
The oral mucosa is one of the first sites to be affected by the SARS-CoV-2. For this reason, healthcare providers performing aerosol-generating procedures (AGPs) in the oral cavity are at high risk of infection with COVID-19. The aim of this systematic review is to verify whether there is evidence in the literature describing a decrease in the salivary viral load of SARS-CoV-2 after using different mouthwashes. An electronic search of the MEDLINE database (via PubMed), Web of Science, SCOPUS, and the Cochrane library database was carried out. The criteria used were those described by the PRISMA® Statement. Randomized controlled trial studies that have used mouthwashes as a form of intervention to reduce the viral load in saliva were included. The risk of bias was analyzed using the Joanna Briggs Institute Critical Appraisal Tool. Ultimately, eight articles were included that met the established criteria. Based on the evidence currently available in the literature, PVP-I, CHX and CPC present significant virucidal activity against SARS-CoV-2 in saliva and could be used as pre-procedural mouthwashes to reduce the risk of cross-infection.
Keywords: COVID-19; SARS-CoV-2; aerosols; cetylpiridinium chloride; chlorhexidine; colony-forming units; hydrogen peroxide; mouthwashes; povidone-iodine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Coronavirus Pandemic (COVID-19) [(accessed on 14 January 2022)]. Available online: https://ourworldindata.org/coronavirus-data.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous