Autophagy Pathways in the Genesis of Plasmodium-Derived Microvesicles: A Double-Edged Sword?
- PMID: 35330166
- PMCID: PMC8955828
- DOI: 10.3390/life12030415
Autophagy Pathways in the Genesis of Plasmodium-Derived Microvesicles: A Double-Edged Sword?
Abstract
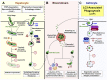
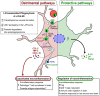
Malaria, caused by Plasmodium species (spp.), is a deadly parasitic disease that results in approximately 400,000 deaths per year globally. Autophagy pathways play a fundamental role in the developmental stages of the parasite within the mammalian host. They are also involved in the production of Plasmodium-derived extracellular vesicles (EVs), which play an important role in the infection process, either by providing nutrients for parasite growth or by contributing to the immunopathophysiology of the disease. For example, during the hepatic stage, Plasmodium-derived EVs contribute to parasite virulence by modulating the host immune response. EVs help in evading the different autophagy mechanisms deployed by the host for parasite clearance. During cerebral malaria, on the other hand, parasite-derived EVs promote an astrocyte-mediated inflammatory response, through the induction of a non-conventional host autophagy pathway. In this review, we will discuss the cross-talk between Plasmodium-derived microvesicles and autophagy, and how it influences the outcome of infection.
Keywords: Plasmodium; astrocytes; autophagy; cerebral malaria; microvesicles; neuroinflammation; pathophysiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organization . World Malaria Report 2020: 20 Years of Global Progress and Challenges. World Health Organization; Geneva, Switzerland: 2020.
-
- Shears M.J., Sekhar Nirujogi R., Swearingen K.E., Renuse S., Mishra S., Jaipal Reddy P., Moritz R.L., Pandey A., Sinnis P. Proteomic Analysis of Plasmodium Merosomes: The Link between Liver and Blood Stages in Malaria. J. Proteome Res. 2019;18:3404–3418. doi: 10.1021/acs.jproteome.9b00324. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

