Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment
- PMID: 35330183
- PMCID: PMC8953753
- DOI: 10.3390/life12030432
Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment
Abstract
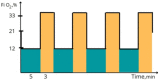
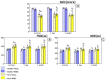
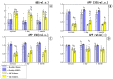
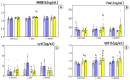
Intermittent hypoxia-hyperoxia training (IHHT) is a non-pharmacological therapeutic modality for management of some chronic- and age-related pathologies, such as Alzheimer’s disease (AD). Our previous studies demonstrated significant improvement of cognitive function after IHHT in the patients with mild cognitive impairment (MCI). The present study further investigated the effects of IHHT on pro-inflammatory factors in healthy elderly individuals and patients with early signs of AD. Twenty-nine subjects (13 healthy subjects without signs of cognitive impairment syndrome and 16 patients diagnosed with MCI; age 52 to 76 years) were divided into four groups: Healthy+Sham (n = 7), Healthy+IHHT (n = 6), MCI+Sham (n = 6), and MCI+IHHT (n = 10). IHHT was carried out 5 days per week for 3 weeks (total 15 sessions), and each daily session included 4 cycles of 5-min hypoxia (12% FIO2) and 3-min hyperoxia (33% FIO2). Decline in cognitive function indices was observed initially in both MCI+Sham and MCI+IHHT groups. The sham training did not alter any of the parameters, whereas IHHT resulted in improvement in latency of cognitive evoked potentials, along with elevation in APP110, GDF15 expression, and MMP9 activity in both healthy subjects and those with MCI. Increased MMP2 activity, HMGB1, and P-selectin expression and decreased NETs formation and Aβ expression were also observed in the MCI+IHHT group. There was a negative correlation between MoCA score and the plasma GDF15 expression (R = −0.5799, p < 0.05) before the initiation of IHHT. The enhanced expression of GDF15 was also associated with longer latency of the event-related potentials P330 and N200 (R = 0.6263, p < 0.05 and R = 0.5715, p < 0.05, respectively). In conclusion, IHHT upregulated circulating levels of some inflammatory markers, which may represent potential triggers for cellular adaptive reprogramming, leading to therapeutic effects against cognitive dysfunction and neuropathological changes during progression of AD. Further investigation is needed to clarify if there is a causative relationship between the improved cognitive function and the elevated inflammatory markers following IHHT.
Keywords: Alzheimer’s disease; cognitive Impairment; hypoxia inducible factor 1; inflammation; intermittent hypoxia; neutrophil extracellular traps.
Conflict of interest statement
E.E. is an owner of CellAir Constructions GmbH, Schorndorf, Germany; L.X. is a co-founder of Xiamen Innovo Medical Technology Co. Ltd., Xiamen, China. These firms had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. All other authors declare no conflict of interests.
Figures



References
-
- Oberlin L.E., Erickson K.I., Mackey R., Klunk W.E., Aizenstein H., Lopresti B.J., Kuller L.H., Lopez O.L., Snitz B.E. Peripheral inflammatory biomarkers predict the deposition and progression of amyloid-beta in cognitively unimpaired older adults. Brain Behav. Immun. 2021;95:178–189. doi: 10.1016/j.bbi.2021.03.015. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

