When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation
- PMID: 35330210
- PMCID: PMC8952681
- DOI: 10.3390/life12030459
When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation
Abstract
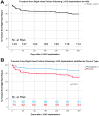
Right heart failure (RHF) is a severe complication after left ventricular assist device (LVAD) implantation. The aim of this study was to analyze the incidence, risk factors, and biomarkers for late RHF including the possible superiority of the device and implantation method. This retrospective, single-center study included patients who underwent LVAD implantation between 2014 and 2018. Primary outcome was freedom from RHF over one-year after LVAD implantation; secondary outcomes included pre- and postoperative risk factors and biomarkers for RHF. Of the 145 consecutive patients (HeartMate 3/HVAD: n = 70/75; female: 13.8%), thirty-one patients (21.4%) suffered RHF after a mean LVAD support of median (IQR) 105 (118) days. LVAD implantation method (less invasive: 46.7% vs. 35.1%, p = 0.29) did not differ significantly in patients with or without RHF, whereas the incidence of RHF was lower in HeartMate 3 vs. HVAD patients (12.9% vs. 29.3%, p = 0.016). Multivariate Cox proportional hazard analysis identified HVAD (HR 4.61, 95% CI 1.12-18.98; p = 0.03), early post-op heart rate (HR 0.96, 95% CI 0.93-0.99; p = 0.02), and central venous pressure (CVP) (HR 1.21, 95% CI 1.05-1.39; p = 0.01) as independent risk factors for RHF, but no association of RHF with increased all-cause mortality (HR 1.00, 95% CI 0.99-1.01; p = 0.50) was found. To conclude, HVAD use, lower heart rate, and higher CVP early post-op were independent risk factors for RHF following LVAD implantation.
Keywords: mechanical circulatory support; right heart failure; risk factor; ventricular assist device.
Conflict of interest statement
T.S. has served as a consultant and advisor for Medtronic Inc. and Abbott Inc., and has received research grants from Medtronic Inc., Abbott Inc., and CorWave. M.G. has served as a consultant for Berlin Heart. H.S. has served as an advisor for Medtronic Inc. and has received research grants from Medtronic Inc. D.W. has served as a proctor and consultant for Abbott Inc. and has served as an advisor for Xenios/Fresenius Medical Care. D.Z. has served as a proctor, advisor, and speaker for Medtronic Inc., Abbott Inc., Berlin Heart, Edwards, Abiomed, and has received research and travel grants from Medtronic Inc. and Abbott Inc. All other authors have nothing to disclose.
Figures
References
-
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

