Diterpenes Specially Produced by Fungi: Structures, Biological Activities, and Biosynthesis (2010-2020)
- PMID: 35330246
- PMCID: PMC8951520
- DOI: 10.3390/jof8030244
Diterpenes Specially Produced by Fungi: Structures, Biological Activities, and Biosynthesis (2010-2020)
Abstract
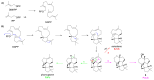
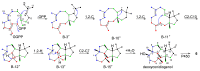
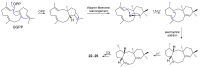
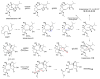
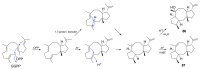
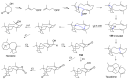
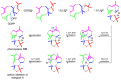
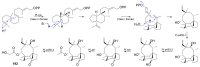
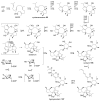
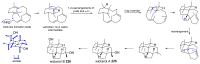
Fungi have traditionally been a very rewarding source of biologically active natural products, while diterpenoids from fungi, such as the cyathane-type diterpenoids from Cyathus and Hericium sp., the fusicoccane-type diterpenoids from Fusicoccum and Alternaria sp., the guanacastane-type diterpenoids from Coprinus and Cercospora sp., and the harziene-type diterpenoids from Trichoderma sp., often represent unique carbon skeletons as well as diverse biological functions. The abundances of novel skeletons, biological activities, and biosynthetic pathways present new opportunities for drug discovery, genome mining, and enzymology. In addition, diterpenoids peculiar to fungi also reveal the possibility of differing biological evolution, although they have similar biosynthetic pathways. In this review, we provide an overview about the structures, biological activities, evolution, organic synthesis, and biosynthesis of diterpenoids that have been specially produced by fungi from 2010 to 2020. We hope this review provides timely illumination and beneficial guidance for future research works of scholars who are interested in this area.
Keywords: biological activity; biosynthesis; diterpenes; isolation; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






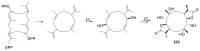
Similar articles
-
Biochemical and Genetic Basis of Guanacastane Diterpene Biosynthesis in Basidiomycete Fungi.Org Lett. 2023 Jul 21;25(28):5345-5349. doi: 10.1021/acs.orglett.3c01924. Epub 2023 Jul 13. Org Lett. 2023. PMID: 37439572
-
Biosynthesis, enzymology, and future of eunicellane diterpenoids.J Ind Microbiol Biotechnol. 2023 Feb 17;50(1):kuad027. doi: 10.1093/jimb/kuad027. J Ind Microbiol Biotechnol. 2023. PMID: 37673680 Free PMC article. Review.
-
The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus Hericium: a review.Chin J Nat Med. 2024 Aug;22(8):676-698. doi: 10.1016/S1875-5364(24)60590-X. Chin J Nat Med. 2024. PMID: 39197960 Review.
-
Structure Diversity, Synthesis, and Biological Activity of Cyathane Diterpenoids in Higher Fungi.Curr Med Chem. 2015;22(19):2375-91. doi: 10.2174/0929867322666150521091333. Curr Med Chem. 2015. PMID: 25994862 Review.
-
Erinacine A and related cyathane diterpenoids: Molecular diversity and mechanisms underlying their neuroprotection and anticancer activities.Pharmacol Res. 2020 Sep;159:104953. doi: 10.1016/j.phrs.2020.104953. Epub 2020 May 30. Pharmacol Res. 2020. PMID: 32485283 Review.
Cited by
-
Unanticipated Large-Scale Deletion in Fusarium graminearum Genome Using CRISPR/Cas9 and Its Impact on Growth and Virulence.J Fungi (Basel). 2023 Jun 14;9(6):673. doi: 10.3390/jof9060673. J Fungi (Basel). 2023. PMID: 37367609 Free PMC article.
-
Secondary Metabolites from Fungi-In Honor of Prof. Dr. Ji-Kai Liu's 60th Birthday.J Fungi (Basel). 2022 Dec 1;8(12):1271. doi: 10.3390/jof8121271. J Fungi (Basel). 2022. PMID: 36547604 Free PMC article.
-
New Tricholomalides D-G from the Mushroom Tricholoma ustaloides Grown in an Italian Beech Wood.Molecules. 2023 Nov 6;28(21):7446. doi: 10.3390/molecules28217446. Molecules. 2023. PMID: 37959864 Free PMC article.
-
Rosthornin B alleviates inflammatory diseases via directly targeting NLRP3.FASEB J. 2024 Dec 13;38(24):e70248. doi: 10.1096/fj.202401198R. FASEB J. 2024. PMID: 39673686 Free PMC article.
-
Exploring the therapeutic potential of diterpenes in gastric cancer: Mechanisms, efficacy, and clinical prospects.Biomol Biomed. 2024 Dec 11;25(1):1-15. doi: 10.17305/bb.2024.10887. Biomol Biomed. 2024. PMID: 39151097 Free PMC article. Review.
References
-
- Dictionary of Natural Products. 2020. [(accessed on 26 August 2021)]. Available online: http://dnp.chemnetbase.com.
-
- Buckingham J., Cooper C.M., Purchase R. Natural Products Desk Reference. CRC Press; Boca Raton, FL, USA: 2016. pp. 1–219.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

