Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk
- PMID: 35330322
- PMCID: PMC8948627
- DOI: 10.3390/jof8030320
Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk
Abstract
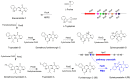
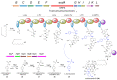
Fungal natural products (NPs) usually possess complicated structures, exhibit satisfactory bioactivities, and are an outstanding source of drug leads, such as the cholesterol-lowering drug lovastatin and the immunosuppressive drug mycophenolic acid. The fungal NPs biosynthetic genes are always arranged within one single biosynthetic gene cluster (BGC). However, a rare but fascinating phenomenon that a crosstalk between two separate BGCs is indispensable to some fungal dimeric NPs biosynthesis has attracted increasing attention. The hybridization of two separate BGCs not only increases the structural complexity and chemical diversity of fungal NPs, but also expands the scope of bioactivities. More importantly, the underlying mechanism for this hybridization process is poorly understood and needs further exploration, especially the determination of BGCs for each building block construction and the identification of enzyme(s) catalyzing the two biosynthetic precursors coupling processes such as Diels-Alder cycloaddition and Michael addition. In this review, we summarized the fungal NPs produced by functional crosstalk of two discrete BGCs, and highlighted their biosynthetic processes, which might shed new light on genome mining for fungal NPs with unprecedented frameworks, and provide valuable insights into the investigation of mysterious biosynthetic mechanisms of fungal dimeric NPs which are constructed by collaboration of two separate BGCs.
Keywords: bioactivity; biosynthetic gene cluster; biosynthetic pathway crosstalk; fungi; natural product; polyketide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




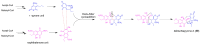



References
Publication types
LinkOut - more resources
Full Text Sources

