Feasibility of Adjuvant Treatment with Abemaciclib-Real-World Data from a Large German Breast Center
- PMID: 35330381
- PMCID: PMC8951288
- DOI: 10.3390/jpm12030382
Feasibility of Adjuvant Treatment with Abemaciclib-Real-World Data from a Large German Breast Center
Abstract
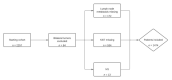
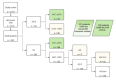
Abemaciclib significantly improves invasive disease-free survival when combined with endocrine therapy in clinical high-risk patients with HR+/Her2- early breast cancer (eBC). The objective of the following study was to model how many patients with eBC would be available for adjuvant treatment with abemaciclib in a real-world setting. Patients that underwent complete surgical treatment for eBC between January 2018 and December 2020 in a large single-center university hospital in Germany were eligible. Descriptive statistics were used to describe the patient population that could benefit from abemaciclib according to the inclusion criteria of monarchE. Of 1474 patients with eBC, 1121 (76.1%) had a HR+/Her2- subtype. Of these, 217 (19.4%) fulfilled the monarchE inclusion criteria. Within patients that fulfilled the monarchE inclusion criteria, 48.9% received no adjuvant or neoadjuvant chemotherapy. Thus, in a real-world situation, fewer patients will be pretreated with chemotherapy than was the case in monarchE. Breast care units are facing a significant patient burden, since the 2-year abemaciclib therapy requires regular monitoring of toxicities. Specific care concepts to strengthen therapy adherence as well as further studies to deescalate adjuvant systemic treatment and individualize CDK 4/6 inhibitor therapy are therefore needed.
Keywords: CDK 4/6; abemaciclib; breast cancer; monarchE; oncology; systemic therapy.
Conflict of interest statement
S.Y.B.: Honoraria from Novartis, Roche, AstraZeneca, Sanofi Aventis. A.D.H.: Speaker and consultancy honoraria from AstraZeneca, Agendia, Exact Sciences Corporation Roche, Novartis, Lilly, MSD, Eisai, Daiichi-Sankyo, Gilead, Teva, Seattle Genetics, and Pfizer. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Moy B., Rumble R.B., Come S.E., Davidson N.E., Di Leo A., Gralow J.R., Hortobagyi G.N., Yee D., Smith I.E., Chavez-MacGregor M., et al. Chemotherapy and Targeted Therapy for Patients with Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer That is Either Endocrine-Pretreated or Hormone Receptor–Negative: ASCO Guideline Update. J. Clin. Oncol. 2021;39:3938–3958. doi: 10.1200/JCO.21.01374. - DOI - PubMed
-
- Schneeweiss A., Ettl J., Lüftner D., Beckmann M.W., Belleville E., Fasching P.A., Fehm T.N., Geberth M., Häberle L., Hadji P., et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer—Data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast. 2020;54:88–95. doi: 10.1016/j.breast.2020.08.011. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

