High Angiotensin-Converting Enzyme and Low Carboxypeptidase N Serum Activity Correlate with Disease Severity in COVID-19 Patients
- PMID: 35330406
- PMCID: PMC8949860
- DOI: 10.3390/jpm12030406
High Angiotensin-Converting Enzyme and Low Carboxypeptidase N Serum Activity Correlate with Disease Severity in COVID-19 Patients
Abstract
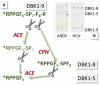
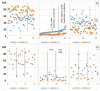
(1) Background: Angiotensin-converting enzyme 2 (ACE2) is a functional receptor of SARS-CoV-2 and counter-balances ACE in the renin-angiotensin system (RAS). An imbalance of the RAS could be associated with severe COVID-19 progression. (2) Methods: Activities of serum proteases angiotensin-converting enzyme (ACE) and carboxypeptidase N (CPN) for 45 hospitalized and 26 convalescent COVID-19 patients were investigated vs. healthy controls using labeled bradykinin (DBK) degradation with and without inhibition by captopril as a read-out. Data were correlated to clinical parameters. (3) Results: DBK degradation and CPN activity were significantly reduced gender-independently in COVID-19 and returned to normal during convalescence. ACE activity was over-active in severe disease progression; product DBK1-5 was significantly increased in critically ill patients and strongly correlated with clinical heart and liver parameters. ACE inhibitors seemed to be protective, as DBK1-5 levels were normal in moderately ill patients in contrast to critically ill patients. (4) Conclusions: CPN and ACE serum activity correlated with disease severity. The RAS is affected in COVID-19, and ACE could be a therapeutic target. Further proof from dedicated studies is needed.
Keywords: ACE; ACE inhibitor; COVID-19; CPN; TLC; bradykinin; corona virus; hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

