Evaluation of CRC-Metastatic Hepatic Lesion Chemoembolization with Irinotecan-Loaded Microspheres, According to the Site of Embolization
- PMID: 35330414
- PMCID: PMC8953829
- DOI: 10.3390/jpm12030414
Evaluation of CRC-Metastatic Hepatic Lesion Chemoembolization with Irinotecan-Loaded Microspheres, According to the Site of Embolization
Abstract
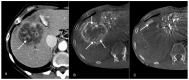
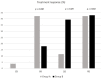
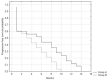
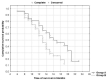
With the chemembolization of colorectal-cancer (CRC)-metastatic hepatic lesions by irinotecan-loaded microspheres, most researchers recommend slow embolizate delivery at the lobar-artery level to the entire liver parenchyma without obtaining visible stasis. An association has been reported between postoperatively visible embolizate stasis and lesion response to treatment. Possibly, in some cases, more selective administration might give greater benefit, particularly with previous systemic chemotherapy failure. Objective: Treatment response evaluation after chemoembolization of CRC-metastatic liver lesions with irinotecan-loaded microspheres, according to a hepatic-artery branch level of administration. Patients and methods: The analysis included 54 patients (24 females, 30 males) with large (median diameter > 5 cm) CRC-metastatic liver lesions, who underwent 196 chemoembolization procedures (mean 3.63 per patient) with irinotecan (100 mg)-loaded microspheres. Patients were divided into two groups according to initial embolizate-administration branch level: Group A (n = 26): at the segmental or subsegmental-vessel level; Group B (n = 28): at the lobar-branch level. Treatment response was assessed by computed-tomography (mRECIST criteria); overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan−Meier method and adverse effects were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0). Results: There were statistically significant differences in the occurrence of partial response (PR): higher in Group A (42.3%) than Group B (17.9%) (p = 0.039) and occurrence of stable disease (SD): lower (p = 0.025) in Group A (11.5%) than Group B (39.4%). However, occurrence of disease progression (PD) was similar: Group A: 42.3%; Group B: 42.9% (p = 0.93). Patients in Group A presented with more favorable PFS (p = 0.029) and OS (p = 0.039) than Group B. Median survival times: Group A: 15.2 months; Group B: 13.1 months. There was no significant difference in complication incidence between groups (Group A: seven complications; Group B: six complications; p = 0.863). Conclusion: Superselective chemoembolizate administration to vessels supplying large CRC-metastatic liver lesions gave better response to treatment and extended patient survival time, without significantly increasing complication risk.
Keywords: DEB-TACE; colorectal cancer; irinotecan; liver chemoembolization; metastases.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures
Similar articles
-
Complications Following Irinotecan-Loaded Microsphere Chemoembolization of Colorectal Metastatic Liver Lesions Associated with Hepatic-Artery Branch Temporary Stasis.Curr Oncol. 2021 Jun 20;28(3):2296-2307. doi: 10.3390/curroncol28030211. Curr Oncol. 2021. PMID: 34203031 Free PMC article.
-
Side Effect/Complication Risk Related to Injection Branch Level of Chemoembolization in Treatment of Metastatic Liver Lesions from Colorectal Cancer.J Clin Med. 2020 Dec 31;10(1):121. doi: 10.3390/jcm10010121. J Clin Med. 2020. PMID: 33396449 Free PMC article.
-
Efficacy and Safety of Liver Chemoembolization Procedures, Combined with FOLFIRI Chemotherapy, in First-Line Treatment of Metastatic Colorectal Cancer in Patients with Oncogene Mutations.Cancers (Basel). 2023 Dec 22;16(1):71. doi: 10.3390/cancers16010071. Cancers (Basel). 2023. PMID: 38201500 Free PMC article.
-
Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases.Cochrane Database Syst Rev. 2020 Mar 12;3(3):CD009498. doi: 10.1002/14651858.CD009498.pub4. Cochrane Database Syst Rev. 2020. PMID: 32163181 Free PMC article.
-
Optimizing the treatment of liver metastases from uveal melanomas with transarterial chemoembolization using melphalan and calibrated microspheres.Bull Cancer. 2020 Dec;107(12):1274-1283. doi: 10.1016/j.bulcan.2020.09.010. Epub 2020 Nov 9. Bull Cancer. 2020. PMID: 33183739 Review.
Cited by
-
Retrospective Analysis of Doses Delivered during Embolization Procedures over the Last 10 Years.J Pers Med. 2022 Oct 12;12(10):1701. doi: 10.3390/jpm12101701. J Pers Med. 2022. PMID: 36294840 Free PMC article.
-
Long noncoding RNA SNHG15: A promising target in human cancers.Front Oncol. 2023 Mar 28;13:1108564. doi: 10.3389/fonc.2023.1108564. eCollection 2023. Front Oncol. 2023. PMID: 37056344 Free PMC article. Review.
References
-
- Reddy S.K., Pawlik T.M., Zorzi D., Gleisner A.L., Ribero D., Assumpcao L., Barbas A.S., Abdalla E.K., Choti M.A., Vauthey J.N. Simultaneous resections of colorectal cancer and synchronous liver metastases: A multi-institutional analysis. Ann. Surg. Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. - DOI - PubMed
-
- Fiorentini G., Aliberti C., Tilli M., Mulazzani L., Graziano F., Giordani P., Mambrini A., Montagnani F., Alessandroni P., Catalano V., et al. Intra-arterial infusion of irinotecan-loaded drug eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) forhepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012;32:1387–1395. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

