A Longitudinal Analysis of SARS-CoV-2 Antibody Responses Among People With HIV
- PMID: 35330585
- PMCID: PMC8940197
- DOI: 10.3389/fmed.2022.768138
A Longitudinal Analysis of SARS-CoV-2 Antibody Responses Among People With HIV
Abstract
Background: The concentration and duration of antibodies (Ab) to SARS-CoV-2 infection predicts the severity of the disease and the clinical outcomes. Older people and those with HIV have impaired immune responses, worse outcomes after SARS-CoV-2 infection, and lower antibody responses after viral infection and vaccination. This study evaluated an Ab response to SARS-CoV-2 in people with HIV (PWH) and without HIV (HIV-) and its association with age.
Methods: A total of 23 COVID+PWH and 21 COVID+HIV- participants were followed longitudinally for 6 months post-mild COVID-19. Immunoglobin G (IgG) and immunoglobin M (IgM) Ab responses were measured by an in-house developed ELISA. Time points and HIV status interaction were analyzed using Poisson generalized estimating equations, and correlations were analyzed using non-parametric tests.
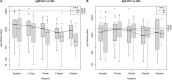
Results: Median age in PWH was 55 years with 28.6% women, while in the HIV- group was 36 years with 60.9% women. The mean time from COVID-19 diagnosis to study enrollment was 16 days for PWH and 11 days for HIV-. The mean CD4+ T-cell count/μl for PWH was 772.10 (±365.21). SARS-CoV-2 IgM and IgG were detected at all time points and Ab response levels did not differ by HIV status (p > 0.05). At entry, age showed a weak direct association with IgG responses (ρ = 0.44, p < 0.05) in HIV- but did not show any association in PWH. Similar associations between age, IgG, and HIV status emerged at day 14 (T1; ρ = 0.50, p < 0.05), 3 months (T3; ρ = 0.50, p < 0.05), and 6 months visit (T4; ρ = 0.78, p < 0.05) in the HIV- group.
Conclusion: The Ab responses in the 6-month post-SARS-CoV-2 infection did not differ by HIV status, though a positive association was found between age and Ab response in older PWH. Results suggest that immune protection and vaccine responses are similar for PWH than for those without HIV infection.
Keywords: COVID-19 vaccine; HIV; SARS-CoV-2; adaptive immunity; antibody; innate immunity.
Copyright © 2022 Alcaide, Nogueira, Salazar, Montgomerie, Rodriguez, Raccamarich, Barreto, McGaugh, Sharkey, Mantero, Rodriguez, Beauchamps and Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Long-lasting adaptive immune memory specific to SARS-CoV-2 in convalescent coronavirus disease 2019 stable people with HIV.AIDS. 2022 Aug 1;36(10):1373-1382. doi: 10.1097/QAD.0000000000003276. Epub 2022 Jun 22. AIDS. 2022. PMID: 35730384
-
High seroconversion rate and SARS-CoV-2 Delta neutralization in people with HIV vaccinated with BNT162b2.AIDS. 2022 Sep 1;36(11):1545-1552. doi: 10.1097/QAD.0000000000003300. Epub 2022 Jun 22. AIDS. 2022. PMID: 35730380
-
Postacute sequelae and adaptive immune responses in people with HIV recovering from SARS-COV-2 infection.AIDS. 2022 Oct 1;36(12):F7-F16. doi: 10.1097/QAD.0000000000003338. Epub 2022 Jul 18. AIDS. 2022. PMID: 35866847 Free PMC article.
-
Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort.Clin Microbiol Infect. 2021 Nov;27(11):1678-1684. doi: 10.1016/j.cmi.2021.06.023. Epub 2021 Jun 26. Clin Microbiol Infect. 2021. PMID: 34186209 Free PMC article. Review.
-
SARS-CoV-2 infection and coronavirus disease 2019 severity in persons with HIV on antiretroviral treatment.AIDS. 2022 Feb 1;36(2):161-168. doi: 10.1097/QAD.0000000000003132. AIDS. 2022. PMID: 34934017 Free PMC article. Review.
Cited by
-
The immune response to SARS-CoV-2 in people with HIV.Cell Mol Immunol. 2024 Feb;21(2):184-196. doi: 10.1038/s41423-023-01087-w. Epub 2023 Oct 11. Cell Mol Immunol. 2024. PMID: 37821620 Free PMC article. Review.
-
Despite delayed kinetics, people living with HIV achieve equivalent antibody function after SARS-CoV-2 infection or vaccination.Front Immunol. 2023 Aug 3;14:1231276. doi: 10.3389/fimmu.2023.1231276. eCollection 2023. Front Immunol. 2023. PMID: 37600825 Free PMC article.
-
A Pilot Single-Blinded, Randomized, Controlled Trial Comparing BNT162b2 vs. JNJ-78436735 Vaccine as the Third Dose After Two Doses of BNT162b2 Vaccine in Solid Organ Transplant Recipients.Transpl Int. 2023 Apr 5;36:10938. doi: 10.3389/ti.2023.10938. eCollection 2023. Transpl Int. 2023. PMID: 37091963 Free PMC article. Clinical Trial.
-
Association between SARS-CoV-2 RNAemia, skewed T cell responses, inflammation, and severity in hospitalized COVID-19 people living with HIV.iScience. 2023 Dec 7;27(1):108673. doi: 10.1016/j.isci.2023.108673. eCollection 2024 Jan 19. iScience. 2023. PMID: 38188525 Free PMC article.
-
Low pre-existing endemic human coronavirus (HCoV-NL63)-specific T cell frequencies are associated with impaired SARS-CoV-2-specific T cell responses in people living with HIV.Front Immunol. 2024 Jan 26;14:1291048. doi: 10.3389/fimmu.2023.1291048. eCollection 2023. Front Immunol. 2024. PMID: 38343437 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

