Experimental and Theoretical Constraints on Amino Acid Formation from PAHs in Asteroidal Settings
- PMID: 35330631
- PMCID: PMC8935471
- DOI: 10.1021/acsearthspacechem.1c00329
Experimental and Theoretical Constraints on Amino Acid Formation from PAHs in Asteroidal Settings
Abstract
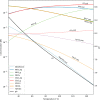
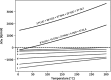
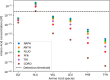
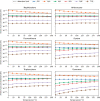
Amino acids and polycyclic aromatic hydrocarbons (PAHs) belong to the range of organic compounds detected in meteorites. In this study, we tested empirically and theoretically if PAHs are precursors for amino acids in carbonaceous chondrites, as previously suggested. We conducted experiments to synthesize amino acids from fluoranthene (PAH), with ammonium bicarbonate as a source for ammonia and carbon dioxide under mimicked asteroidal conditions. In our thermodynamic calculations, we extended our analysis to additional PAH-amino acid combinations. We explored 36 reactions involving the PAHs naphthalene, anthracene, fluoranthene, pyrene, triphenylene, and coronene and the amino acids glycine, alanine, valine, leucine, phenylalanine, and tyrosine. Our experiments do not show the formation of amino acids, whereas our theoretical results hint that PAHs could be precursors of amino acids in carbonaceous chondrites at low temperatures.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Amino-acid synthesis in carbonaceous meteorites by aqueous alteration of polycyclic aromatic hydrocarbons.Nature. 1990 Feb 22;343(6260):728-31. doi: 10.1038/343728a0. Nature. 1990. PMID: 11536464
-
Deuterium Isotope Fractionation of Polycyclic Aromatic Hydrocarbons in Meteorites as an Indicator of Interstellar/Protosolar Processing History.Life (Basel). 2022 Sep 1;12(9):1368. doi: 10.3390/life12091368. Life (Basel). 2022. PMID: 36143402 Free PMC article.
-
Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay.Mutat Res. 2001 Oct 18;497(1-2):49-62. doi: 10.1016/s1383-5718(01)00240-6. Mutat Res. 2001. PMID: 11525907
-
Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air.Environ Health Perspect. 2002 Jun;110 Suppl 3(Suppl 3):451-88. doi: 10.1289/ehp.110-1241197. Environ Health Perspect. 2002. PMID: 12060843 Free PMC article. Review.
-
Synthesis of Polycyclic Aromatic Hydrocarbons by Phenyl Addition-Dehydrocyclization: The Third Way.Angew Chem Int Ed Engl. 2019 Nov 25;58(48):17442-17450. doi: 10.1002/anie.201909876. Epub 2019 Oct 16. Angew Chem Int Ed Engl. 2019. PMID: 31482662 Review.
Cited by
-
Understanding Titan's Prebiotic Chemistry: Synthesizing Amino Acids Through Aminonitrile Alkaline Hydrolysis.ACS Earth Space Chem. 2024 Nov 20;8(12):2380-2392. doi: 10.1021/acsearthspacechem.4c00114. eCollection 2024 Dec 19. ACS Earth Space Chem. 2024. PMID: 39720226 Free PMC article.
References
-
- Martins Z. Organic chemistry of carbonaceous meteorites. Elements 2011, 7 (1), 35–40. 10.2113/gselements.7.1.35. - DOI
-
- Botta O.; Bada J. L. Extraterrestrial Organic Compounds in Meteorites. Surveys in Geophysics 2002, 23 (5), 411–467. 10.1023/A:1020139302770. - DOI
-
- Glavin D. P.; Alexander C. M. D.; Aponte J. C.; Dworkin J. P.; Elsila J. E.; Yabuta H., The Origin and Evolution of Organic Matter in Carbonaceous Chondrites and Links to Their Parent Bodies. In Primitive Meteorites and Asteroids; Elsevier, 2018.
LinkOut - more resources
Full Text Sources
