In situ injectable hydrogel-loaded drugs induce anti-tumor immune responses in melanoma immunochemotherapy
- PMID: 35330634
- PMCID: PMC8938887
- DOI: 10.1016/j.mtbio.2022.100238
In situ injectable hydrogel-loaded drugs induce anti-tumor immune responses in melanoma immunochemotherapy
Abstract
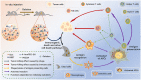
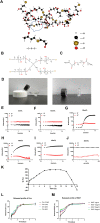
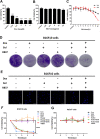
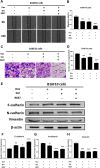
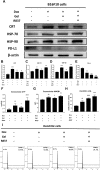
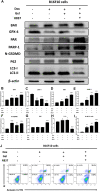
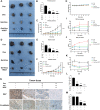
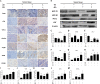
Melanoma is a highly aggressive tumor located in the skin, with limited traditional therapies. In order to reduce the side effects caused by traditional administration method and amplify the killing effect of immune system against tumor cells, an in situ injectable hydrogel drug delivery system is developed for the first time which co-delivers doxorubicin (Dox) and imiquimod (R837) for the synergistic therapy of melanoma. The mechanical properties and stability of the hydrogel are characterized and the optimal doses of hydrogel and drugs are also identified. As a result, the co-delivery system effectively suppresses melanoma growth and metastatic progression both in vitro and in vivo. Further studies show that the co-delivery system causes immunogenic cell death, activation of antigen presenting cells, comprising dendritic cells and M1 macrophages, and secretion of related cytokines consisted of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), subsequently with the activation of T lymphocytes and natural killer cells in spleen and tumor area. The co-delivery system also decreases the suppressive immune responses, including infiltration of M2 macrophages and secretion of interleukin-10 (IL-10), in vivo. Besides, other death modes are induced by the co-delivery system, including apoptosis and non-apoptotic cell death. In a word, this co-delivery system induces melanoma cell death directly and activates immune system for further tumor killing simultaneously, which shows probability for precise targeted tumor therapy.
Keywords: Anti-tumor immune response; Cell death; Immunogenic cell death; Injectable hydrogel; Melanoma.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
An injectable, in situ forming and NIR-responsive hydrogel persistently reshaping tumor microenvironment for efficient melanoma therapy.Biomater Res. 2023 Nov 19;27(1):118. doi: 10.1186/s40824-023-00462-y. Biomater Res. 2023. PMID: 37981704 Free PMC article.
-
Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy.Theranostics. 2019 Apr 12;9(8):2299-2314. doi: 10.7150/thno.30577. eCollection 2019. Theranostics. 2019. PMID: 31149045 Free PMC article.
-
Design of an Injectable Polypeptide Hydrogel Depot Containing the Immune Checkpoint Blocker Anti-PD-L1 and Doxorubicin to Enhance Antitumor Combination Therapy.Macromol Biosci. 2021 Jun;21(6):e2100049. doi: 10.1002/mabi.202100049. Epub 2021 Apr 19. Macromol Biosci. 2021. PMID: 33871152
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment.Bioact Mater. 2020 Dec 26;6(7):1973-1987. doi: 10.1016/j.bioactmat.2020.12.010. eCollection 2021 Jul. Bioact Mater. 2020. PMID: 33426371 Free PMC article. Review.
Cited by
-
The Role of TRL7/8 Agonists in Cancer Therapy, with Special Emphasis on Hematologic Malignancies.Vaccines (Basel). 2023 Jan 28;11(2):277. doi: 10.3390/vaccines11020277. Vaccines (Basel). 2023. PMID: 36851155 Free PMC article. Review.
-
Application of Nanoparticles in the Diagnosis and Treatment of Colorectal Cancer.Anticancer Agents Med Chem. 2024;24(18):1305-1326. doi: 10.2174/0118715206323900240807110122. Anticancer Agents Med Chem. 2024. PMID: 39129164 Free PMC article. Review.
-
Targeting AKT induced Ferroptosis through FTO/YTHDF2-dependent GPX4 m6A methylation up-regulating and degradating in colorectal cancer.Cell Death Discov. 2023 Dec 15;9(1):457. doi: 10.1038/s41420-023-01746-x. Cell Death Discov. 2023. PMID: 38102129 Free PMC article.
-
Hydrogel drug delivery systems for minimally invasive local immunotherapy of cancer.Adv Drug Deliv Rev. 2023 Nov;202:115083. doi: 10.1016/j.addr.2023.115083. Epub 2023 Sep 9. Adv Drug Deliv Rev. 2023. PMID: 37673217 Free PMC article. Review.
-
Programmable Release of Chemotherapeutics from Ferrocene-Based Injectable Hydrogels Slows Melanoma Growth.Adv Healthc Mater. 2024 Oct;13(27):e2400265. doi: 10.1002/adhm.202400265. Epub 2024 Jul 15. Adv Healthc Mater. 2024. PMID: 39007274 Free PMC article.
References
-
- Holmes D. The cancer that rises with the sun. Nature. 2014;515:S110–S111. - PubMed
-
- Ambrosi L., Khan S., Carvajal R.D., Yang J. Novel targets for the treatment of melanoma. Curr. Oncol. Rep. 2019;21:97. - PubMed
-
- Wada-Ohno M., Ito T., Furue M. Adjuvant therapy for melanoma. Curr. Treat. Options Oncol. 2019;20:63. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

