Projection-specific Activity of Layer 2/3 Neurons Imaged in Mouse Primary Somatosensory Barrel Cortex During a Whisker Detection Task
- PMID: 35330741
- PMCID: PMC8788860
- DOI: 10.1093/function/zqaa008
Projection-specific Activity of Layer 2/3 Neurons Imaged in Mouse Primary Somatosensory Barrel Cortex During a Whisker Detection Task
Erratum in
-
Corrigendum to: Projection-specific Activity of Layer 2/3 Neurons Imaged in Mouse Primary Somatosensory Barrel Cortex During a Whisker Detection Task.Function (Oxf). 2020 Oct 6;1(2):zqaa020. doi: 10.1093/function/zqaa020. eCollection 2020. Function (Oxf). 2020. PMID: 35333036 Free PMC article.
Abstract
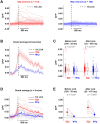
The brain processes sensory information in a context- and learning-dependent manner for adaptive behavior. Through reward-based learning, relevant sensory stimuli can become linked to execution of specific actions associated with positive outcomes. The neuronal circuits involved in such goal-directed sensory-to-motor transformations remain to be precisely determined. Studying simple learned sensorimotor transformations in head-restrained mice offers the opportunity for detailed measurements of cellular activity during task performance. Here, we trained mice to lick a reward spout in response to a whisker deflection and an auditory tone. Through two-photon calcium imaging of retrogradely labeled neurons, we found that neurons located in primary whisker somatosensory barrel cortex projecting to secondary whisker somatosensory cortex had larger calcium signals than neighboring neurons projecting to primary whisker motor cortex in response to whisker deflection and auditory stimulation, as well as before spontaneous licking. Longitudinal imaging of the same neurons revealed that these projection-specific responses were relatively stable across 3 days. In addition, the activity of neurons projecting to secondary whisker somatosensory cortex was more highly correlated than for neurons projecting to primary whisker motor cortex. The large and correlated activity of neurons projecting to secondary whisker somatosensory cortex might enhance the pathway-specific signaling of important sensory information contributing to task execution. Our data support the hypothesis that communication between primary and secondary somatosensory cortex might be an early critical step in whisker sensory perception. More generally, our data suggest the importance of investigating projection-specific neuronal activity in distinct populations of intermingled excitatory neocortical neurons during task performance.
Keywords: goal-directed behavior; licking; neocortex; projection neurons; sensorimotor transformation; sensory perception; somatosensory cortex; two-photon calcium imaging; whisker sensation.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Physiological Society 2020.
Figures

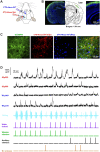
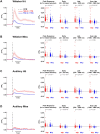
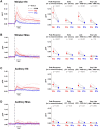
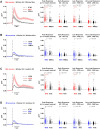



Comment in
-
Pathway-Specific Activation in Sensorimotor Cortical Networks: Perspective on "Projection-Specific Activity of Layer 2/3 Neurons Imaged in Mouse Primary Somatosensory Barrel Cortex During a Whisker Detection Task".Function (Oxf). 2020 Jul 16;1(1):zqaa011. doi: 10.1093/function/zqaa011. eCollection 2020. Function (Oxf). 2020. PMID: 35330740 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

