Cell Type-Specific Membrane Potential Changes in Dorsolateral Striatum Accompanying Reward-Based Sensorimotor Learning
- PMID: 35330797
- PMCID: PMC8788857
- DOI: 10.1093/function/zqab049
Cell Type-Specific Membrane Potential Changes in Dorsolateral Striatum Accompanying Reward-Based Sensorimotor Learning
Abstract
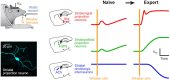
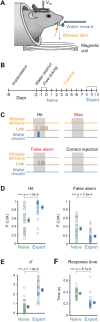
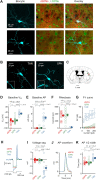
The striatum integrates sensorimotor and motivational signals, likely playing a key role in reward-based learning of goal-directed behavior. However, cell type-specific mechanisms underlying reinforcement learning remain to be precisely determined. Here, we investigated changes in membrane potential dynamics of dorsolateral striatal neurons comparing naïve mice and expert mice trained to lick a reward spout in response to whisker deflection. We recorded from three distinct cell types: (i) direct pathway striatonigral neurons, which express type 1 dopamine receptors; (ii) indirect pathway striatopallidal neurons, which express type 2 dopamine receptors; and (iii) tonically active, putative cholinergic, striatal neurons. Task learning was accompanied by cell type-specific changes in the membrane potential dynamics evoked by the whisker deflection and licking in successfully-performed trials. Both striatonigral and striatopallidal types of striatal projection neurons showed enhanced task-related depolarization across learning. Striatonigral neurons showed a prominent increase in a short latency sensory-evoked depolarization in expert compared to naïve mice. In contrast, the putative cholinergic striatal neurons developed a hyperpolarizing response across learning, driving a pause in their firing. Our results reveal cell type-specific changes in striatal membrane potential dynamics across the learning of a simple goal-directed sensorimotor transformation, helpful for furthering the understanding of the various potential roles of different basal ganglia circuits.
Keywords: goal-directed sensorimotor transformation; reward-based learning; striatum; whole-cell membrane potential.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Physiological Society.
Figures

Comment in
-
Getting Excited About Learning.Function (Oxf). 2021;2(6):zqab059. doi: 10.1093/function/zqab059. Epub 2021 Nov 9. Function (Oxf). 2021. PMID: 35252871 Free PMC article. No abstract available.
Similar articles
-
Dopamine dynamics in nucleus accumbens across reward-based learning of goal-directed whisker-to-lick sensorimotor transformations in mice.Heliyon. 2024 Sep 11;10(18):e37831. doi: 10.1016/j.heliyon.2024.e37831. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323852 Free PMC article.
-
Striatal Dopamine Signals and Reward Learning.Function (Oxf). 2023 Oct 3;4(6):zqad056. doi: 10.1093/function/zqad056. eCollection 2023. Function (Oxf). 2023. PMID: 37841525 Free PMC article. Review.
-
Cell-Type-Specific Sensorimotor Processing in Striatal Projection Neurons during Goal-Directed Behavior.Neuron. 2015 Oct 21;88(2):298-305. doi: 10.1016/j.neuron.2015.08.039. Epub 2015 Oct 1. Neuron. 2015. PMID: 26439527 Free PMC article.
-
Target-specific membrane potential dynamics of neocortical projection neurons during goal-directed behavior.Elife. 2016 Jun 21;5:e15798. doi: 10.7554/eLife.15798. Elife. 2016. PMID: 27328320 Free PMC article.
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
Cited by
-
Cell-type-specific auditory responses in the striatum are shaped by feedforward inhibition.Cell Rep. 2025 Jan 28;44(1):115090. doi: 10.1016/j.celrep.2024.115090. Epub 2024 Dec 24. Cell Rep. 2025. PMID: 39721025 Free PMC article.
-
Dopamine dynamics in nucleus accumbens across reward-based learning of goal-directed whisker-to-lick sensorimotor transformations in mice.Heliyon. 2024 Sep 11;10(18):e37831. doi: 10.1016/j.heliyon.2024.e37831. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323852 Free PMC article.
-
Striatal Dopamine Signals and Reward Learning.Function (Oxf). 2023 Oct 3;4(6):zqad056. doi: 10.1093/function/zqad056. eCollection 2023. Function (Oxf). 2023. PMID: 37841525 Free PMC article. Review.
-
Getting Excited About Learning.Function (Oxf). 2021;2(6):zqab059. doi: 10.1093/function/zqab059. Epub 2021 Nov 9. Function (Oxf). 2021. PMID: 35252871 Free PMC article. No abstract available.
-
Unraveling the dynamics of dopamine release and its actions on target cells.Trends Neurosci. 2023 Mar;46(3):228-239. doi: 10.1016/j.tins.2022.12.005. Epub 2023 Jan 10. Trends Neurosci. 2023. PMID: 36635111 Free PMC article. Review.
References
-
- Arber S, Costa RM. Connecting neuronal circuits for movement. Science. 2018;360(6396):1403–1404. - PubMed
-
- Park J, Coddington LT, Dudman JT. Basal ganglia circuits for action specification. Annu Rev Neurosci. 2020;43(1):485–507. - PubMed
-
- Klaus A, Alves da Silva J, Costa RMW. If, and when to move: basal ganglia circuits and self-paced action initiation. Annu Rev Neurosci. 2019;42(1):459–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
