Mitochondrial P2X7 Receptor Localization Modulates Energy Metabolism Enhancing Physical Performance
- PMID: 35330818
- PMCID: PMC8788778
- DOI: 10.1093/function/zqab005
Mitochondrial P2X7 Receptor Localization Modulates Energy Metabolism Enhancing Physical Performance
Erratum in
-
Corrigendum to Sarti et al. Mitochondrial P2X7 Receptor Localization Modulates Energy Metabolism Enhancing Physical Performance.Function (Oxf). 2021 Jun 18;2(4):zqab025. doi: 10.1093/function/zqab025. eCollection 2021. Function (Oxf). 2021. PMID: 35333037 Free PMC article.
Abstract
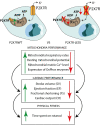
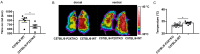
Basal expression of the P2X7 receptor (P2X7R) improves mitochondrial metabolism, Adenosine 5'-triphosphate (ATP) synthesis, and overall fitness of immune and non-immune cells. We investigated P2X7R contribution to energy metabolism and subcellular localization in fibroblasts (mouse embryo fibroblasts and HEK293 human fibroblasts), mouse microglia (primary brain microglia, and the N13 microglia cell line), and heart tissue. The P2X7R localizes to mitochondria, and its lack (1) decreases basal respiratory rate, ATP-coupled respiration, maximal uncoupled respiration, resting mitochondrial potential, mitochondrial matrix Ca2+ level, (2) modifies expression pattern of oxidative phosphorylation enzymes, and (3) severely affects cardiac performance. Hearts from P2rx7-deleted versus wild-type mice are larger, heart mitochondria smaller, and stroke volume, ejection fraction, fractional shortening, and cardiac output, are significantly decreased. Accordingly, the physical fitness of P2X7R-null mice is severely reduced. Thus, the P2X7R is a key modulator of mitochondrial energy metabolism and a determinant of physical fitness.
Keywords: P2X7; dilated cardiomyopathy; extracellular ATP; mitochondria; oxidative phosphorylation; purinergic signaling; respiratory chain.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Physiological Society.
Figures






Comment in
-
Mitochondrial Localization and Function of the Purinergic Receptor P2X7.Function (Oxf). 2021 Jan 28;2(2):zqab006. doi: 10.1093/function/zqab006. eCollection 2021. Function (Oxf). 2021. PMID: 35330813 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
