Involvement of the Dorsal Vagal Complex in Alcohol-Related Behaviors
- PMID: 35330845
- PMCID: PMC8940294
- DOI: 10.3389/fnbeh.2022.801825
Involvement of the Dorsal Vagal Complex in Alcohol-Related Behaviors
Abstract
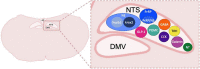
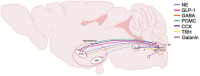
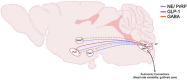
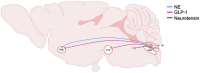
The neurobiological mechanisms that regulate the development and maintenance of alcohol use disorder (AUD) are complex and involve a wide variety of within and between systems neuroadaptations. While classic reward, preoccupation, and withdrawal neurocircuits have been heavily studied in terms of AUD, viable treatment targets from this established literature have not proven clinically effective as of yet. Therefore, examination of additional neurocircuitries not classically studied in the context of AUD may provide novel therapeutic targets. Recent studies demonstrate that various neuropeptides systems are important modulators of alcohol reward, seeking, and intake behaviors. This includes neurocircuitry within the dorsal vagal complex (DVC), which is involved in the control of the autonomic nervous system, control of intake of natural rewards like food, and acts as a relay of interoceptive sensory information via interactions of numerous gut-brain peptides and neurotransmitter systems with DVC projections to central and peripheral targets. DVC neuron subtypes produce a variety of neuropeptides and transmitters and project to target brain regions critical for reward such as the mesolimbic dopamine system as well as other limbic areas important for the negative reinforcing and aversive properties of alcohol withdrawal such as the extended amygdala. This suggests the DVC may play a role in the modulation of various aspects of AUD. This review summarizes the current literature on neurotransmitters and neuropeptides systems in the DVC (e.g., norepinephrine, glucagon-like peptide 1, neurotensin, cholecystokinin, thyrotropin-releasing hormone), and their potential relevance to alcohol-related behaviors in humans and rodent models for AUD research. A better understanding of the role of the DVC in modulating alcohol related behaviors may lead to the elucidation of novel therapeutic targets for drug development in AUD.
Keywords: alcohol use disorder; gut-brain axis; interoception; nucleus of the tractus solitarius; vagus nerve.
Copyright © 2022 Keller, Hajnal, Browning, Arnold and Silberman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit/Stress Surfeit Disorder.Curr Top Behav Neurosci. 2023 Jul 9. doi: 10.1007/7854_2023_424. Online ahead of print. Curr Top Behav Neurosci. 2023. PMID: 37421551
-
The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies.Front Pharmacol. 2023 Mar 30;14:1063033. doi: 10.3389/fphar.2023.1063033. eCollection 2023. Front Pharmacol. 2023. PMID: 37063267 Free PMC article. Review.
-
Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1.Brain Res. 2020 Jan 15;1727:146562. doi: 10.1016/j.brainres.2019.146562. Epub 2019 Nov 21. Brain Res. 2020. PMID: 31759971 Review.
-
Food-intake dysregulation in type 2 diabetic Goto-Kakizaki rats: hypothesized role of dysfunctional brainstem thyrotropin-releasing hormone and impaired vagal output.Neuroscience. 2013 Sep 5;247:43-54. doi: 10.1016/j.neuroscience.2013.05.017. Epub 2013 May 20. Neuroscience. 2013. PMID: 23701881 Free PMC article.
-
Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function.J Comp Neurol. 1997 Jan 6;377(1):49-69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. J Comp Neurol. 1997. PMID: 8986872
Cited by
-
The integrative process promoted by EMDR in dissociative disorders: neurobiological mechanisms, psychometric tools, and intervention efficacy on the psychological impact of the COVID-19 pandemic.Front Psychol. 2023 Sep 1;14:1164527. doi: 10.3389/fpsyg.2023.1164527. eCollection 2023. Front Psychol. 2023. PMID: 37727746 Free PMC article. Review.
-
Ethanol inhibits pancreatic projecting neurons in the dorsal motor nucleus of the vagus.Brain Res Bull. 2022 Oct 15;189:121-129. doi: 10.1016/j.brainresbull.2022.08.020. Epub 2022 Aug 23. Brain Res Bull. 2022. PMID: 35998791 Free PMC article. Review.
-
Central amygdala neuroimmune signaling in alcohol use disorder.Addict Neurosci. 2025 Mar;14:100194. doi: 10.1016/j.addicn.2024.100194. Epub 2024 Dec 21. Addict Neurosci. 2025. PMID: 40336623 Free PMC article.
-
Neurotensin and Alcohol Use Disorders: Towards a Pharmacological Treatment.Int J Mol Sci. 2023 May 12;24(10):8656. doi: 10.3390/ijms24108656. Int J Mol Sci. 2023. PMID: 37240004 Free PMC article. Review.
-
Emerging pharmacological targets for alcohol use disorder.Alcohol. 2024 Dec;121:103-114. doi: 10.1016/j.alcohol.2024.07.007. Epub 2024 Jul 26. Alcohol. 2024. PMID: 39069210 Review.
References
-
- Alonso G., Szafarczyk A., Balmefrézol M., Assenmacher I. (1986). Immunocytochemical evidence for stimulatory control by the ventral noradrenergic bundle of parvocellular neurons of the paraventricular nucleus secreting corticotropin releasing hormone and vasopressin in rats. Brain Res. 397 297–307. 10.1016/0006-8993(86)90631-1 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

