Elevated CO2 Improves the Physiology but Not the Final Yield in Spring Wheat Genotypes Subjected to Heat and Drought Stress During Anthesis
- PMID: 35330869
- PMCID: PMC8940247
- DOI: 10.3389/fpls.2022.824476
Elevated CO2 Improves the Physiology but Not the Final Yield in Spring Wheat Genotypes Subjected to Heat and Drought Stress During Anthesis
Abstract
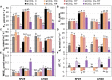
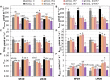
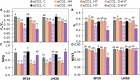
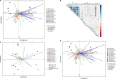
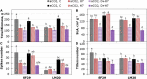
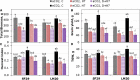
Heat and drought events often occur concurrently as a consequence of climate change and have a severe impact on crop growth and yield. Besides, the accumulative increase in the atmospheric CO2 level is expected to be doubled by the end of this century. It is essential to understand the consequences of climate change combined with the CO2 levels on relevant crops such as wheat. This study evaluated the physiology and metabolite changes and grain yield in heat-sensitive (SF29) and heat-tolerant (LM20) wheat genotypes under individual heat stress or combined with drought applied during anthesis at ambient (aCO2) and elevated CO2 (eCO2) levels. Both genotypes enhanced similarly the WUE under combined stresses at eCO2. However, this increase was due to different stress responses, whereas eCO2 improved the tolerance in heat-sensitive SF29 by enhancing the gas exchange parameters, and the accumulation of compatible solutes included glucose, fructose, β-alanine, and GABA to keep water balance; the heat-tolerant LM20 improved the accumulation of phosphate and sulfate and reduced the lysine metabolism and other metabolites including N-acetylornithine. These changes did not help the plants to improve the final yield under combined stresses at eCO2. Under non-stress conditions, eCO2 improved the yield of both genotypes. However, the response differed among genotypes, most probably as a consequence of the eCO2-induced changes in glucose and fructose at anthesis. Whereas the less-productive genotype LM20 reduced the glucose and fructose and increased the grain dimension as the effect of the eCO2 application, the most productive genotype SF29 increased the two carbohydrate contents and ended with higher weight in the spikes. Altogether, these findings showed that the eCO2 improves the tolerance to combined heat and drought stress but not the yield in spring wheat under stress conditions through different mechanisms. However, under non-stress conditions, it could improve mainly the yield to the less-productive genotypes. Altogether, the results demonstrated that more studies focused on the combination of abiotic stress are needed to understand better the spring wheat responses that help the identification of genotypes more resilient and productive under these conditions for future climate conditions.
Keywords: chlorophyll fluorescence; elevated CO2; gas exchange; grain yield; heat stress; targeted metabolomic analysis; wheat.
Copyright © 2022 Abdelhakim, Mendanha, Palma, Vrobel, Štefelová, Ćavar Zeljković, Tarkowski, De Diego, Wollenweber, Rosenqvist and Ottosen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer PC declared a shared affiliation with one of the authors ER to the handling editor at the time of the review.
Figures

References
-
- Abdelhakim L. O. A., Palma C. F. F., Zhou R., Wollenweber B., Ottosen C. O., Rosenqvist E. (2021). The effect of individual and combined drought and heat stress under elevated CO2 on physiological responses in spring wheat genotypes. Plant Physiol. Biochem. 162 301–314. 10.1016/j.plaphy.2021.02.015 - DOI - PubMed
-
- Akter N., Islam M. R. (2017). Heat stress effects and management in wheat. a review. Agron. Sustain. Dev. 37:37.
-
- Amthor J. S. (2001). Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. Field Crop. Res. 73 1–34. 10.1016/s0378-4290(01)00179-4 - DOI
LinkOut - more resources
Full Text Sources

