Intravenous N-Acetylcysteine in Management of COVID-19: A Case Series
- PMID: 35331045
- PMCID: PMC8958286
- DOI: 10.1177/08971900221080283
Intravenous N-Acetylcysteine in Management of COVID-19: A Case Series
Abstract
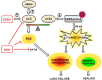
A novel coronavirus, severe acute respiratory syndrome coronavirus-2, was isolated from patients' lower respiratory tracts in December 2019. As of May 19, 2021, there were over 33 million reported infections and almost 600,000 deaths in the United States. The infection, coronavirus disease-19 (COVID-19), can lead to cytokine storm, with elevations in interleukin-6 (IL-6), IL-10, tumor necrosis factor-α, nuclear factor-kappaB (NF-kappaB), and glutathione reductase. NF-kappaB activation is necessary for further transcription of other pro-inflammatory markers. Glutathione may play a role in modulation of NF-kappaB activation and elevated glutathione reductase may indicate glutathione depletion. Administration of N-acetylcysteine (NAC) may replenish spent glutathione and attenuate over-activation of NF-kappaB. This retrospective case series included 10 patients who were COVID-19 positive and received intravenous NAC in an attempt to attenuate the cytokine storm. Patients' outcomes were graded based on the World Health Organization symptom severity scale from 0, no evidence of infection, to 8, death. Overall, the median WHO Scale prior to NAC was 6.5, and increased by day seven, which indicated clinical worsening. This retrospective case series showed no benefit of NAC; however, further studies are needed to elucidate if differences in drug regimens would lead to positive results.
Keywords: COVID-19; N-acetylcysteine; coronavirus; cytokine storm; glutathione.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

