Effects of auricular acupressure on chemotherapy-induced nausea and vomiting in breast cancer patients: a preliminary randomized controlled trial
- PMID: 35331208
- PMCID: PMC8953362
- DOI: 10.1186/s12906-022-03543-y
Effects of auricular acupressure on chemotherapy-induced nausea and vomiting in breast cancer patients: a preliminary randomized controlled trial
Abstract
Background: Auricular acupressure (AA) has been viewed as a promising approach to managing chemotherapy-induced nausea and vomiting (CINV) but relevant research evidence has been inconclusive. This study aimed to examine the effects of AA on CINV in breast cancer (BC) patients undergoing chemotherapy.
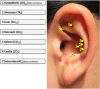
Methods: A preliminary randomized controlled trial was conducted in 114 BC patients. Participants were randomly allocated to a true AA group (n = 38), a sham AA group (n = 38), and a standard care group (n = 38). All the participants were provided with standard antiemetic treatment and care, while the true AA group and the sham AA group received an additional 5-day true AA and a 5-day sham AA, respectively. Acute and delayed CINV were assessed by using the MASCC Antiemesis Tool (MAT), anticipatory nausea and vomiting were measured by the Index of Nausea, Vomiting, and Retching (INVR), and patients' quality of life (QoL) was evaluated by the Functional Assessment of Cancer Therapy-Breast (FACT-B).
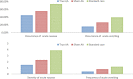
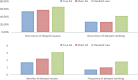
Results: Both the true and sham AA groups reported improved CINV outcomes than the standard care group, with the true AA demonstrating larger effects than the sham comparison. The true and sham AA groups had higher complete response (CR) rates of CINV when compared with the standard care group, with the difference in the CR of acute CINV achieving statistical significance (p = 0.03). Both the true and sham AA groups demonstrated lower incidence and severity of acute CINV compared with the standard care group with the among-group difference reaching statistical significance for the occurrence (p = 0.04) and severity (p = 0.001) of acute nausea. No significant differences in anticipatory CINV and QoL were found among the groups.
Conclusion: The use of AA plus standard antiemetic treatment and care was superior to the use of standard antiemetic treatment and care alone in managing CINV among BC patients receiving chemotherapy. The antiemetic effects of AA were identified to be more profound in improving acute CINV, particularly acute nausea. The antiemetic effects of AA were deemed to be a mixture of specific treatment effects and placebo effects, and the placebo effects were very large and even reached clinical significance.
Trial registration: ClinicalTrials.gov; NCT02403037 ; Registered March 31, 2015.
Keywords: Auricular therapy; Chemotherapy; Nausea and vomiting; Neoplasms; Randomized controlled trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Escobar Y, Cajaraville G, Virizuela JA, Álvarez R, Muñoz A, Olariaga O, Martínez P. Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer. 2015;23(9):2833–2840. - PMC - PubMed
-
- Hsieh RK, Chan A, Kim HK, Yu S, Kim JG, Lee MA, Keefe DM. Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer. 2015;23(1):263–272. - PubMed
-
- Molassiotis A, Lee PH, Burke TA, Dicato M, Gascon P, Roila F, Aapro M. Anticipatory nausea, risk factors, and its impact on chemotherapy-induced nausea and vomiting: Results from the Pan European Emesis Registry study. J Pain Symptom Manage. 2016;51(6):987–993. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

