Saliva collection via capillary method may underestimate arboviral transmission by mosquitoes
- PMID: 35331315
- PMCID: PMC8944160
- DOI: 10.1186/s13071-022-05198-7
Saliva collection via capillary method may underestimate arboviral transmission by mosquitoes
Abstract
Background: Arthropod-borne viruses (arboviruses) impose a major health and economic burden on human populations globally, with mosquitoes serving as important vectors. Measuring the ability of a mosquito population to transmit an arbovirus is important in terms of evaluating its public health risk. In the laboratory, a variety of methods are used to estimate arboviral transmission by mosquitoes, including indirect methods involving viral detection from mosquito saliva collected by forced salivation. The accuracy of indirect methods to estimate arbovirus transmission to live animal hosts has not been fully evaluated.
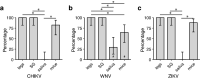
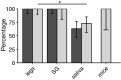
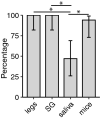
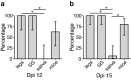
Methods: We compared three commonly used proxies of arboviral transmission, namely, the presence of virus in mosquito legs, in salivary glands (SG) and in saliva collected in capillary tubes using forced salivation, with direct transmission estimates from mosquitoes to suckling mice. We analyzed five vector-virus combinations, including Aedes aegypti infected with chikungunya virus, West Nile virus and Zika virus; Culex quinquefasciatus infected with West Nile virus; and Aedes triseriatus infected with La Crosse virus.
Results: Comparatively, the methods of detecting virus infection in mosquito legs and in SG were equally accurate in predicting transmission. Overall, the presence of virus in mosquito legs was a more accurate predictor of transmission than the commonly implemented viral detection method using forced salivation into a capillary tube, and was subject to less technical variation.
Conclusions: These results suggest that, in general, forced salivation methods tend to underestimate virus transmission, and they provide confidence in the use of mosquito leg screens to evaluate the transmission potential of a mosquito population.
Keywords: Arbovirus; Proxy; Saliva; Transmission; Vector.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Bidirectional Interactions between Arboviruses and the Bacterial and Viral Microbiota in Aedes aegypti and Culex quinquefasciatus.mBio. 2022 Oct 26;13(5):e0102122. doi: 10.1128/mbio.01021-22. Epub 2022 Sep 7. mBio. 2022. PMID: 36069449 Free PMC article.
-
Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo.J Virol. 2019 Aug 28;93(18):e00705-19. doi: 10.1128/JVI.00705-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243123 Free PMC article.
-
Not all mosquitoes are created equal: A synthesis of vector competence experiments reinforces virus associations of Australian mosquitoes.PLoS Negl Trop Dis. 2022 Oct 4;16(10):e0010768. doi: 10.1371/journal.pntd.0010768. eCollection 2022 Oct. PLoS Negl Trop Dis. 2022. PMID: 36194577 Free PMC article.
-
Aedes Mosquito Salivary Components and Their Effect on the Immune Response to Arboviruses.Front Cell Infect Microbiol. 2020 Aug 7;10:407. doi: 10.3389/fcimb.2020.00407. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850501 Free PMC article. Review.
-
Antiviral Compounds for Blocking Arboviral Transmission in Mosquitoes.Viruses. 2021 Jan 14;13(1):108. doi: 10.3390/v13010108. Viruses. 2021. PMID: 33466915 Free PMC article. Review.
Cited by
-
Jamestown Canyon virus is transmissible by Aedes aegypti and is only moderately blocked by Wolbachia co-infection.PLoS Negl Trop Dis. 2023 Sep 5;17(9):e0011616. doi: 10.1371/journal.pntd.0011616. eCollection 2023 Sep. PLoS Negl Trop Dis. 2023. PMID: 37669272 Free PMC article.
-
Invasive Aedes albopictus is a competent vector for O'nyong Nyong virus.One Health. 2025 May 4;20:101062. doi: 10.1016/j.onehlt.2025.101062. eCollection 2025 Jun. One Health. 2025. PMID: 40630106 Free PMC article.
-
Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes.Trop Med Infect Dis. 2024 Sep 2;9(9):201. doi: 10.3390/tropicalmed9090201. Trop Med Infect Dis. 2024. PMID: 39330890 Free PMC article.
-
Assessing Urban Yellow Fever Transmission Risk: Aedes aegypti Vector Competence in Argentina.Viruses. 2025 May 16;17(5):718. doi: 10.3390/v17050718. Viruses. 2025. PMID: 40431729 Free PMC article.
-
Aedes aegypti VLG-1 challenges the assumed antiviral nature of Vago genes.BMC Biol. 2025 Jul 28;23(1):223. doi: 10.1186/s12915-025-02325-5. BMC Biol. 2025. PMID: 40717062 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

