Exosomal CagA from Helicobacter pylori aggravates intestinal epithelium barrier dysfunction in chronic colitis by facilitating Claudin-2 expression
- PMID: 35331316
- PMCID: PMC8944046
- DOI: 10.1186/s13099-022-00486-0
Exosomal CagA from Helicobacter pylori aggravates intestinal epithelium barrier dysfunction in chronic colitis by facilitating Claudin-2 expression
Abstract
Background: The chronic infection with Helicobacter pylori (H. pylori), especially cytotoxin-associated gene A-positive (CagA+) strains, has been associated with various extragastric disorders. Evaluating the potential impacts of virulence factor CagA on intestine may provide a better understanding of H. pylori pathogenesis such as colitis. The intestinal mucosal barrier is essential for maintaining its integrity and functions. However, how persistent CagA+ H. pylori colonization influences barrier disruption and thereby affects chronic colitis is not fully understood.
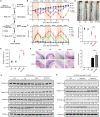
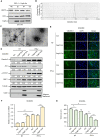
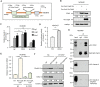
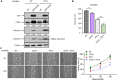
Results: Chronic colitis models of CagA+ H. pylori-colonized mice treated with 2% Dextran sulphate sodium (DSS) were established to assess the disease activity and pertinent expression of tight junction proteins closely related to mucosal integrity. The aggravating effect of CagA+ H. pylori infection on DSS-induced chronic colitis was confirmed in mouse models. In addition, augmented Claudin-2 expression was detected in CagA+ H. pylori infection conditions and selected for mechanistic analysis. Next, GES-1 human gastric epithelial cells were cultured with CagA+ H. pylori or a recombinant CagA protein, and exosomes isolated from conditioned media were then identified. We assessed the Claudin-2 levels after exposure to CagA+ exosomes, CagA- exosomes, and IFN-γ incubation, revealing that CagA+ H. pylori compromised the colonic mucosal barrier and facilitated IFN-γ-induced intestinal epithelial destruction through CagA-containing exosome-mediated mechanisms. Specifically, CagA upregulated Claudin-2 expression at the transcriptional level via a CDX2-dependent mechanism to slow the restoration of wounded mucosa in colitis in vitro.
Conclusions: These data suggest that exosomes containing CagA facilitate CDX2-dependent Claudin-2 maintenance. The exosome-dependent mechanisms of CagA+ H. pylori infection are indispensable for damaging the mucosal barrier integrity in chronic colitis, which may provide a new idea for inflammatory bowel disease (IBD) treatment.
Keywords: CDX2; CagA; Claudin-2; Colitis; Exosome; Tight junction.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. - PubMed
-
- Fox JG, Wang TC. Helicobacter pylori infection: pathogenesis. Curr Opin Gastroenterol. 2002;18:15–25. - PubMed
-
- Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. - PubMed
Grants and funding
- 82002614/National Natural Science Foundation of China
- 2021JJ40935/Natural Science Foundation of Hunan Province
- YX202103/the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University
- 1053320183950/the Fundamental Research Funds of Central South University
- 201310533059/the National Innovative Training Program of China
LinkOut - more resources
Full Text Sources

