Spatial organization of the kelp microbiome at micron scales
- PMID: 35331334
- PMCID: PMC8944128
- DOI: 10.1186/s40168-022-01235-w
Spatial organization of the kelp microbiome at micron scales
Abstract
Background: Elucidating the spatial structure of host-associated microbial communities is essential for understanding taxon-taxon interactions within the microbiota and between microbiota and host. Macroalgae are colonized by complex microbial communities, suggesting intimate symbioses that likely play key roles in both macroalgal and bacterial biology, yet little is known about the spatial organization of microbes associated with macroalgae. Canopy-forming kelp are ecologically significant, fixing teragrams of carbon per year in coastal kelp forest ecosystems. We characterized the micron-scale spatial organization of bacterial communities on blades of the kelp Nereocystis luetkeana using fluorescence in situ hybridization and spectral imaging with a probe set combining phylum-, class-, and genus-level probes to localize and identify > 90% of the microbial community.
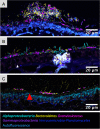
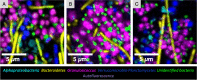
Results: We show that kelp blades host a dense microbial biofilm composed of disparate microbial taxa in close contact with one another. The biofilm is spatially differentiated, with clustered cells of the dominant symbiont Granulosicoccus sp. (Gammaproteobacteria) close to the kelp surface and filamentous Bacteroidetes and Alphaproteobacteria relatively more abundant near the biofilm-seawater interface. A community rich in Bacteroidetes colonized the interior of kelp tissues. Microbial cell density increased markedly along the length of the kelp blade, from sparse microbial colonization of newly produced tissues at the meristematic base of the blade to an abundant microbial biofilm on older tissues at the blade tip. Kelp from a declining population hosted fewer microbial cells compared to kelp from a stable population.
Conclusions: Imaging revealed close association, at micrometer scales, of different microbial taxa with one another and with the host. This spatial organization creates the conditions necessary for metabolic exchange among microbes and between host and microbiota, such as provisioning of organic carbon to the microbiota and impacts of microbial nitrogen metabolisms on host kelp. The biofilm coating the surface of the kelp blade is well-positioned to mediate interactions between the host and surrounding organisms and to modulate the chemistry of the surrounding water column. The high density of microbial cells on kelp blades (105-107 cells/cm2), combined with the immense surface area of kelp forests, indicates that biogeochemical functions of the kelp microbiome may play an important role in coastal ecosystems. Video abstract.
Keywords: Biogeography; CLASI-FISH; Endophytic; Epiphytic; Host-microbe; Nereocystis luetkeana; Polymicrobial interaction; Spatial structure.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Functional Insights into the Kelp Microbiome from Metagenome-Assembled Genomes.mSystems. 2022 Jun 28;7(3):e0142221. doi: 10.1128/msystems.01422-21. Epub 2022 Jun 1. mSystems. 2022. PMID: 35642511 Free PMC article.
-
Successional Dynamics and Seascape-Level Patterns of Microbial Communities on the Canopy-Forming Kelps Nereocystis luetkeana and Macrocystis pyrifera.Front Microbiol. 2019 Feb 26;10:346. doi: 10.3389/fmicb.2019.00346. eCollection 2019. Front Microbiol. 2019. PMID: 30863387 Free PMC article.
-
Oxygen metabolism shapes microbial settlement on photosynthetic kelp blades compared to artificial kelp substrates.Environ Microbiol Rep. 2021 Apr;13(2):176-184. doi: 10.1111/1758-2229.12923. Epub 2020 Dec 28. Environ Microbiol Rep. 2021. PMID: 33372322
-
Impact of kelp forest on seawater chemistry - A review.Mar Pollut Bull. 2023 Nov;196:115655. doi: 10.1016/j.marpolbul.2023.115655. Epub 2023 Oct 13. Mar Pollut Bull. 2023. PMID: 37839130 Review.
-
Biogeography of the Oral Microbiome: The Site-Specialist Hypothesis.Annu Rev Microbiol. 2019 Sep 8;73:335-358. doi: 10.1146/annurev-micro-090817-062503. Epub 2019 Jun 10. Annu Rev Microbiol. 2019. PMID: 31180804 Free PMC article. Review.
Cited by
-
The Diversity and Functional Capacity of Microbes Associated with Coastal Macrophytes.mSystems. 2022 Oct 26;7(5):e0059222. doi: 10.1128/msystems.00592-22. Epub 2022 Aug 22. mSystems. 2022. PMID: 35993708 Free PMC article.
-
Functional Insights into the Kelp Microbiome from Metagenome-Assembled Genomes.mSystems. 2022 Jun 28;7(3):e0142221. doi: 10.1128/msystems.01422-21. Epub 2022 Jun 1. mSystems. 2022. PMID: 35642511 Free PMC article.
-
Coral holobiont research needs spatial analyses at the microbial scale.Environ Microbiol. 2023 Jan;25(1):179-183. doi: 10.1111/1462-2920.16237. Epub 2022 Nov 21. Environ Microbiol. 2023. PMID: 36209397 Free PMC article. No abstract available.
-
Why biofouling cannot contribute to the vertical transport of small microplastic.Microplast nanoplast. 2024;4(1):19. doi: 10.1186/s43591-024-00098-2. Epub 2024 Oct 7. Microplast nanoplast. 2024. PMID: 39385966 Free PMC article. Review.
-
The Cultured Microbiome of Pollinated Maize Silks Shifts after Infection with Fusarium graminearum and Varies by Distance from the Site of Pathogen Inoculation.Pathogens. 2023 Nov 6;12(11):1322. doi: 10.3390/pathogens12111322. Pathogens. 2023. PMID: 38003787 Free PMC article.
References
-
- Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T. The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol Rev. 2013;37(3):462–476. - PubMed
-
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438(7064):90–93. - PubMed
-
- Weigel BL, Pfister CA. The dynamics and stoichiometry of dissolved organic carbon release by kelp. Ecology. 2021;102(2):e03221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

