Effect of antenatal magnesium sulphate on MRI biomarkers of white matter development at term equivalent age: The MagNUM Study
- PMID: 35331677
- PMCID: PMC9043972
- DOI: 10.1016/j.ebiom.2022.103923
Effect of antenatal magnesium sulphate on MRI biomarkers of white matter development at term equivalent age: The MagNUM Study
Abstract
Background: Magnesium sulphate given to women prior to very preterm birth protects the perinatal brain, so fewer babies die or develop cerebral palsy. How magnesium sulphate exerts these beneficial effects remains uncertain. The MagNUM Study aimed to assess the effect of exposure to antenatal magnesium sulphate on MRI measures of brain white matter microstructure at term equivalent age.
Methods: Nested cohort study within the Magnesium sulphate at 30 to <34 weeks' Gestational age Neuroprotection Trial (MAGENTA). Australian New Zealand Clinical Trials Registry ACTRN12611000491965. Mothers at risk of preterm birth at 30 to <34 weeks' gestation were randomised to receive either 4 g of magnesium sulphate heptahydrate [8 mmol magnesium ions], or saline placebo, when preterm birth was planned or expected within 24 h. Participating babies underwent diffusion tensor MRI at term equivalent age. The main outcomes were fractional anisotropy across the white matter tract skeleton compared using Tract-based Spatial Statistics (TBSS), with adjustment for postmenstrual age at birth and at MRI, and MRI site. Researchers and families were blind to treatment group allocation during data collection and analyses.
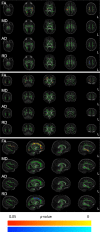
Findings: Of the 109 babies the demographics of the 49 babies exposed to magnesium sulphate were similar to the 60 babies exposed to placebo. In babies whose mothers were allocated to magnesium sulphate, fractional anisotropy was lower within the corticospinal tracts and corona radiata, the superior and inferior longitudinal fasciculi, and the inferior fronto-occipital fasciculi compared to babies whose mothers were allocated placebo (P < 0·05).
Interpretation: In babies born preterm after 30 weeks' gestation, antenatal magnesium sulphate exposure did not promote development of white matter microstructure in pathways affecting motor or cognitive function. This suggests that if the neuroprotective effect of magnesium sulphate treatment prior to preterm birth is confirmed at this gestation, the mechanisms are not related to accelerated white matter maturation inferred from fractional anisotropy.
Funding: This study was funded by a project grant from the Health Research Council of New Zealand (HRC 14/153).
Keywords: Antenatal magnesium sulphate; Brain development; Cerebral palsy; Diffusion tensor magnetic resonance imaging; Fetal neuroprotection; Preterm birth.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures


Retracted and republished from
-
Effect of antenatal magnesium sulphate on MRI biomarkers of white matter development at term equivalent age: The magnum study.EBioMedicine. 2020 Sep;59:102957. doi: 10.1016/j.ebiom.2020.102957. Epub 2020 Aug 25. EBioMedicine. 2020. Retracted and republished in: EBioMedicine. 2022 Apr;78:103923. doi: 10.1016/j.ebiom.2022.103923. Retraction in: EBioMedicine. 2022 Apr;78:103966. doi: 10.1016/j.ebiom.2022.103966. PMID: 32858399 Free PMC article. Retracted and republished. Retracted.
References
-
- World Health Organization . World Health Organization; Geneva: 2015. Recommendations on Interventions to Improve Preterm Birth Outcomes.http://www.who.int/reproductivehealth/publications/maternal_perinatal_he... - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

