Coeliac plexus radiosurgery for pain management in patients with advanced cancer : study protocol for a phase II clinical trial
- PMID: 35332036
- PMCID: PMC8948399
- DOI: 10.1136/bmjopen-2021-050169
Coeliac plexus radiosurgery for pain management in patients with advanced cancer : study protocol for a phase II clinical trial
Abstract
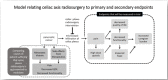
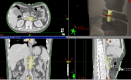
Introduction: Pancreatic cancer is characterised by severe mid-back and epigastric pain caused by tumour invasion of the coeliac nerve plexus. This pain is often poorly managed with standard treatments. This clinical trial investigates a novel approach in which high-dose radiation (radiosurgery) is targeted to the retroperitoneal coeliac plexus nerve bundle. Preliminary results from a single institution pilot trial are promising: pain relief is substantial and side effects minimal. The goals of this study are to validate these findings in an international multisetting, and investigate the impact on quality of life and functional status among patients with terminal cancer.
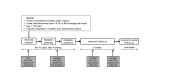
Methods and analysis: A single-arm prospective phase II clinical trial. Eligible patients are required to have severe coeliac pain of at least five on the 11-point BPI average pain scale and Eastern Cooperative Oncology Group performance status of two or better. Non-pancreatic cancers invading the coeliac plexus are also eligible. The intervention involves irradiating the coeliac plexus using a single fraction of 25 Gy. The primary endpoint is the complete or partial pain response at 3 weeks. Secondary endpoints include pain at 6 weeks, analgesic use, hope, qualitative of life, caregiver burden and functional outcomes, all measured using validated instruments. The protocol is expected to open at a number of cancer centres across the globe, and a quality assurance programme is included. The protocol requires that 90 evaluable patients" be accrued, based upon the assumption that a third of patients are non-evaluable (e.g. due to death prior to 3-weeks post-treatment assessment, or spontaneous improvement of pain pre-treatment), it is estimated that a total of 120 patients will need to be accrued. Supported by Gateway for Cancer Research and the Israel Cancer Association.
Ethics and dissemination: Ethic approval for this study has been obtained at eight academic medical centres located across the Middle East, North America and Europe. Results will be disseminated through conference presentations and peer-reviewed publications.
Trial registration number: NCT03323489.
Keywords: adult palliative care; cancer pain; pain management; radiation oncology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
