HydroZitLa inhibits calcium oxalate stone formation in nephrolithic rats and promotes longevity in nematode Caenorhabditis elegans
- PMID: 35332173
- PMCID: PMC8948263
- DOI: 10.1038/s41598-022-08316-8
HydroZitLa inhibits calcium oxalate stone formation in nephrolithic rats and promotes longevity in nematode Caenorhabditis elegans
Abstract
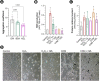
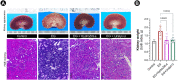
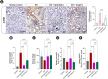
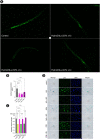
Low fluid intake, low urinary citrate excretion, and high oxidative stress are main causative factors of calcium oxalate (CaOx) nephrolithiasis. HydroZitLa contains citrate and natural antioxidants and is developed to correct these three factors simultaneously. Antioxidants theoretically can prolong the lifespan of organisms. In this study, we preclinically investigated the antilithogenic, lifespan-extending and anti-aging effects of HydroZitLa in HK-2 cells, male Wistar rats, and Caenorhabditis elegans. HydroZitLa significantly inhibited CaOx crystal aggregation in vitro and reduced oxidative stress in HK-2 cells challenged with lithogenic factors. For experimental nephrolithiasis, rats were divided into four groups: ethylene glycol (EG), EG + HydroZitLa, EG + Uralyt-U, and untreated control. CaOx deposits in kidneys of EG + HydroZitLa and EG + Uralyt-U rats were significantly lower than those of EG rats. Intrarenal expression of 4-hydroxynonenal in EG + HydroZitLa rats was significantly lower than that of EG rats. The urinary oxalate levels of EG + HydroZitLa and EG + Uralyt-U rats were significantly lower than those of EG rats. The urinary citrate levels of EG + HydroZitLa and EG + Uralyt-U rats were restored to the level in normal control rats. In C. elegans, HydroZitLa supplementation significantly extended the median lifespan of nematodes up to 34% without altering feeding ability. Lipofuscin accumulation in HydroZitLa-supplemented nematodes was significantly lower than that of non-supplemented control. Additionally, HydroZitLa inhibited telomere shortening, p16 upregulation, and premature senescence in HK-2 cells exposed to lithogenic stressors. Conclusions, HydroZitLa inhibited oxidative stress and CaOx formation both in vitro and in vivo. HydroZitLa extended the lifespan and delayed the onset of aging in C. elegans and human kidney cells. This preclinical evidence suggests that HydroZitLa is beneficial for inhibiting CaOx stone formation, promoting longevity, and slowing down aging.
© 2022. The Author(s).
Conflict of interest statement
C.B., N.C. and N.L. are inventors of HydroZitLa. Chulalongkorn University and the inventors own the intellectual property for HydroZitLa. None of the other authors have any conflict of interest to declare in relation to the present work.
Figures


References
-
- Shah J, Whitfield HN. Urolithiasis through the ages. BJU Int. 2002;89:801–810. - PubMed
-
- Boonla C, Hunapathed C, Bovornpadungkitti S, Poonpirome K, Tungsanga K, Sampatanukul P, Tosukhowong P. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008;101:1170–1177. - PubMed
-
- Boonla C, Krieglstein K, Bovornpadungkitti S, Strutz F, Spittau B, Predanon C, Tosukhowong P. Fibrosis and evidence for epithelial-mesenchymal transition in the kidneys of patients with staghorn calculi. BJU Int. 2011;108:1336–1345. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

