LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma
- PMID: 35332278
- PMCID: PMC9061296
- DOI: 10.1038/s41375-022-01531-2
LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma
Abstract
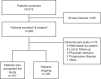
Despite treatment advances, patients with multiple myeloma (MM) often progress through standard drug classes including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies (mAbs). LocoMMotion (ClinicalTrials.gov identifier: NCT04035226) is the first prospective study of real-life standard of care (SOC) in triple-class exposed (received at least a PI, IMiD, and anti-CD38 mAb) patients with relapsed/refractory MM (RRMM). Patients (N = 248; ECOG performance status of 0-1, ≥3 prior lines of therapy or double refractory to a PI and IMiD) were treated with median 4.0 (range, 1-20) cycles of SOC therapy. Overall response rate was 29.8% (95% CI: 24.2-36.0). Median progression-free survival (PFS) and median overall survival (OS) were 4.6 (95% CI: 3.9-5.6) and 12.4 months (95% CI: 10.3-NE). Treatment-emergent adverse events (TEAEs) were reported in 83.5% of patients (52.8% grade 3/4). Altogether, 107 deaths occurred, due to progressive disease (n = 74), TEAEs (n = 19), and other reasons (n = 14). The 92 varied regimens utilized demonstrate a lack of clear SOC for heavily pretreated, triple-class exposed patients with RRMM in real-world practice and result in poor outcomes. This supports a need for new treatments with novel mechanisms of action.
© 2022. The Author(s).
Conflict of interest statement
M-VM: honoraria/member on the board of directors/advisory committees (Amgen, Adaptive Biotechnologies, BMS Celgene, Janssen, Oncopeptides, Pfizer, Regeneron, Roche, Sanofi, Sea-Gen, Takeda). KW: honoraria (Roche); consultant/honoraria (Adaptive Biotech, Karyopharm, Takeda); honoraria/membership on board of directors/advisory committees (BMS, Celgene, Amgen, GSK, Janssen, Oncopeptides, Sanofi). VDS: honoraria (Abbvie, Alexion, Amgen, AOP Pharmaceutical, BMS Celgene, Grifols, GSK, Sanofi, SOBI, Takeda); honoraria/research funding (Novartis). HG: honoraria (GSK); consultant (Adaptive Biotech); research funding (Incyte, Johns Hopkins University, Molecular Partners, MSD, Mundipharma); honoraria/research funding (Novartis); honoraria/research funding (Chugai); consultant/ honoraria/research funding (Amgen, BMS, Celgene, Janssen, Sanofi). MD: honoraria/research funding (Amgen, Celgene, Janssen, Sanofi). MM: honoraria (Amgen, Astellas, BMS, Gilead, Takeda, Novartis, Pfizer, Adaptive Biotech); honoraria/research funding (Celgene, Sanofi). MC: honoraria (Novartis); honoraria/consultant (GSK, Adaptive Biotech); honoraria/consultant, membership on board of directors/advisory committees/speakers bureau (BMS, Sanofi, AbbVie, Amgen, Takeda); honoraria/consultant/member of board of directors/advisory and speakers bureaus/travel accommodations and expenses (Janssen, Celgene). DD: employee/consultant/ honoraria/member of board of directors/advisory committees (Janssen Cilag); member of board of directors/advisory committees/research funding (Celgene). AuP: honoraria (AbbVie, Amgen, BMS Celgene, GSK); honoraria/research funding (Sanofi); honoraria/member of board of directors/advisory committees (Janssen); honoraria/research funding/member of board of directors/advisory committees (Takeda). RB: honoraria/research funding (Allogene, Janssen, BMS, Gilead, Servier). NWCJvdD: research funding (Cellectis), consultant (Takeda, Roche, Novartis, Bayer, Servier); research funding/consultant (Janssen, Amgen); consultant/honoraria (BMS Celgene). EMO: consultant (Sanofi, Oncopeptides); consultant/honoraria (Janssen, Takeda); consultant/honoraria/research funding (Amgen, BMS Celgene). ChS: honoraria (AbbVie); consultant (Roche); honoraria/member of board of directors/advisory committees (Novartis, Takeda); consultant/honoraria/member of board of directors/advisory committees (Amgen, BMS, Janssen). FG: honoraria (BMS); consultant/honoraria (Sanofi); member of board of directors/advisory committees (Roche, Adaptive Biotech, Oncopeptides, Bluebird Bio); honoraria/member of board of directors/advisory committees (Amgen, Celgene, Janssen, Takeda, AbbVie, GSK). PR-O: honoraria/speaker bureaus (BMS Celgene, Janssen, Amgen, GSK, Sanofi, Regeneron, Oncopeptides). AB: honoraria (Amgen, BMS Celgene, Janssen, Sanofi). AnP, JMS: employees and holders of stock options at Janssen. CaS, MS, RW: employees of Janssen. SK: employee of Janssen R&D. VS: employee of Janssen Pharmaceutica NV. MV: employee of Janssen Global Services, LLC. TN: employee of Legend Biotech USA. JS-M: consultant/member of board of directors/advisory committees (AbbVie, Amgen, BMS Celgene, GSK, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, Secura Bio, Takeda). PS: honoraria/research funding (SkylineDx); consultant/honoraria/research (Amgen, BMS Celgene, Janssen, Karyopharm, Takeda). PM: honoraria (AbbVie, Amgen, Janssen, Sanofi, Celgene/BMS, Oncopeptides). RV, JL-H, EA, WR, and HE: no relationships to disclose. Funded by Janssen Research & Development, LLC and Legend Biotech, Inc.; ClinicalTrials.gov identifier, NCT04035226.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

