Cardiovascular efficacy of liraglutide and semaglutide in individuals with diabetes and peripheral artery disease
- PMID: 35332654
- PMCID: PMC9325529
- DOI: 10.1111/dom.14700
Cardiovascular efficacy of liraglutide and semaglutide in individuals with diabetes and peripheral artery disease
Abstract
Aim: To evaluate the cardiovascular (CV) efficacy of liraglutide and semaglutide in patients with type 2 diabetes (T2D) and peripheral artery disease (PAD).
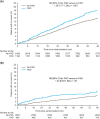
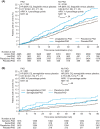
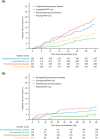
Materials and methods: LEADER and SUSTAIN 6 trials investigated subcutaneous liraglutide (≤1.8 mg/day) and semaglutide (0.5 or 1.0 mg/week), respectively, versus placebo in patients with T2D and high CV risk (median follow-up: 3.8 and 2.1 years, respectively). The primary outcome was a composite of CV death, non-fatal myocardial infarction or non-fatal stroke (major adverse CV event [MACE]) according to the presence of PAD at baseline.
Results: Overall, 1184/9340 (12.7%) patients in LEADER and 460/3297 (14.0%) in SUSTAIN 6 had PAD at baseline. Patients with PAD were at an ~35% increased risk of MACE versus those without (LEADER: hazard ratio [HR] 1.36, 95% confidence interval [CI] 1.17-1.58; SUSTAIN 6: HR 1.33, 95% CI 0.94-1.83). The effects of both therapies on MACE were consistently beneficial in patients with PAD (liraglutide: HR 0.77, 95% CI 0.58-1.01; semaglutide: 0.61, 0.33-1.13) and without (liraglutide: HR 0.89, 95% CI 0.79-1.00; semaglutide: HR 0.77, 95% CI 0.58-1.01; Pinteraction = .34 for liraglutide and .49 for semaglutide). Absolute risk reductions for MACE were higher in patients with PAD (liraglutide: 4.13%-point, 95% CI -0.15-8.42; semaglutide: 4.63%-point, 95% CI -0.58-9.84) versus without (liraglutide:1.42%-point, 95% CI -0.03-2.87; semaglutide: 1.90%-point, 95% CI 0.00-3.80).
Conclusion: Both liraglutide and semaglutide reduce MACE with consistent CV efficacy regardless of PAD status.
Trial registration: ClinicalTrials.gov NCT01179048 NCT01720446.
Keywords: GLP-1; cardiovascular disease; macrovascular disease; peripheral artery disease; receptor agonists; type 2 diabetes.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
SV holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharma, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization. MA‐O has received consulting fees from Amgen and Bayer. LAL has received research funding (to institution), provided continuing medical education on behalf of and/or acted as an adviser to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lexicon, Merck, Novo Nordisk, Pfizer, Sanofi and Servier. CDM has received consulting fees from Amgen, AstraZeneca, BioAge, Boehringer Ingelheim and PhaseBio. SR is an employee and shareholder of Novo Nordisk A/S. HAS is a former employee of Novo Nordisk A/S. MSR is an employee and shareholder of Novo Nordisk A/S. MPB is the Executive Director of CPC, a non‐profit academic research organization affiliated with the University of Colorado that receives research grant/consulting funding from Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women's Hospital, Bristol‐Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, EverlyWell, Faraday, Fortress. Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, Medtronic, Moderna, Novate Medical, Novo Nordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Ciosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth, BioTherapeutics, University of Colorado, Worldwide Clinical Trials, Wraser and Yale Cardiovascular Research Group. MPB also reports being a scientific advisor for the STRIDE Trial (supported by Novo Nordisk).
Figures

References
-
- American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333‐3341. - PubMed
-
- Silbernagel G, Rein P, Saely CH, et al. Prevalence of type 2 diabetes is higher in peripheral artery disease than in coronary artery disease patients. Diab Vasc Dis Res. 2015;12:146‐149. - PubMed
-
- Khoury H, Lavoie L, Welner S, Folkerts K. The burden of major adverse cardiac events and antiplatelet prevention in patients with coronary or peripheral arterial disease. Cardiovasc Ther. 2016;34:115‐124. - PubMed
-
- Verma S, Mazer CD, Al‐Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA‐REG OUTCOME. Circulation. 2018;137:405‐407. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

