Efficacy and Safety of a Krabbe Disease Gene Therapy
- PMID: 35333110
- PMCID: PMC9142772
- DOI: 10.1089/hum.2021.245
Efficacy and Safety of a Krabbe Disease Gene Therapy
Abstract
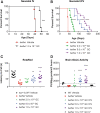
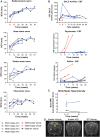
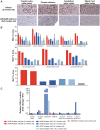
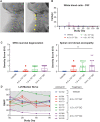
Krabbe disease is a lysosomal storage disease caused by mutations in the gene that encodes galactosylceramidase, in which galactosylsphingosine (psychosine) accumulation drives demyelination in the central and peripheral nervous systems, ultimately progressing to death in early childhood. Gene therapy, alone or in combination with transplant, has been developed for almost two decades in mouse models, with increasing therapeutic benefit paralleling the improvement of next-generation adeno-associated virus (AAV) vectors. This effort has recently shown remarkable efficacy in the canine model of the disease by two different groups that used either systemic or cerebrospinal fluid (CSF) administration of AAVrh10 or AAV9. Building on our experience developing CSF-delivered, AAV-based drug products for a variety of neurodegenerative disorders, we conducted efficacy, pharmacology, and safety studies of AAVhu68 delivered to the CSF in two relevant natural Krabbe animal models, and in nonhuman primates. In newborn Twitcher mice, the highest dose (1 × 1011 genome copies [GC]) of AAVhu68.hGALC injected into the lateral ventricle led to a median survival of 130 days compared to 40.5 days in vehicle-treated mice. When this dose was administered intravenously, the median survival was 49 days. A single intracisterna magna injection of AAVhu68.cGALC at 3 × 1013 GC into presymptomatic Krabbe dogs increased survival for up to 85 weeks compared to 12 weeks in controls. It prevented psychosine accumulation in the CSF, preserved peripheral nerve myelination, ambulation, and decreased brain neuroinflammation and demyelination, although some regions remained abnormal. In a Good Laboratory Practice-compliant toxicology study, we administered the clinical candidate into the cisterna magna of 18 juvenile rhesus macaques at 3 doses that displayed efficacy in mice. We observed no dose-limiting toxicity and sporadic minimal degeneration of dorsal root ganglia (DRG) neurons. Our studies demonstrate the efficacy, scalability, and safety of a single cisterna magna AAVhu68 administration to treat Krabbe disease. ClinicalTrials.Gov ID: NCT04771416.
Keywords: AAV; Krabbe; Twitcher mouse; cisterna magna; demyelination; galactosylceramidase (GALC); galactosylceramide; lysosomal storage disease; psychosine.
Conflict of interest statement
J.M.W. is a paid advisor to and holds equity in iECURE, Scout Bio, Passage Bio, and the Center for Breakthrough Medicines (CBM). He also holds equity in the G2 Bio-associated asset companies. He has sponsored research agreements with Amicus Therapeutics, Biogen, CBM, Elaaj Bio, FA212, G2 Bio, G2 Bio-associated asset companies, iECURE, Janssen, Passage Bio, and Scout Bio, which are licensees of Penn technology. J.M.W. and J.H. are inventors on patents that have been licensed to various biopharmaceutical companies and for which they may receive payments.
A.M.B. is a beneficiary of a licensing agreement with Axovant Gene Therapies (royalties); has received income from Neurogene (consulting and honorarium); and is an inventor on a patent application (Gray SJ, Lykken E, Vite CH, Bradbury AM.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
