A kinase-dead Csf1r mutation associated with adult-onset leukoencephalopathy has a dominant inhibitory impact on CSF1R signalling
- PMID: 35333324
- PMCID: PMC9002114
- DOI: 10.1242/dev.200237
A kinase-dead Csf1r mutation associated with adult-onset leukoencephalopathy has a dominant inhibitory impact on CSF1R signalling
Abstract
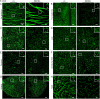
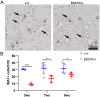
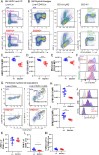
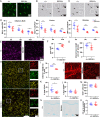
Amino acid substitutions in the kinase domain of the human CSF1R gene are associated with autosomal dominant adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). To model the human disease, we created a disease-associated mutation (pGlu631Lys; E631K) in the mouse Csf1r locus. Homozygous mutation (Csf1rE631K/E631K) phenocopied the Csf1r knockout, with prenatal mortality or severe postnatal growth retardation and hydrocephalus. Heterozygous mutation delayed the postnatal expansion of tissue macrophage populations in most organs. Bone marrow cells from Csf1rE631K/+mice were resistant to CSF1 stimulation in vitro, and Csf1rE631K/+ mice were unresponsive to administration of a CSF1-Fc fusion protein, which expanded tissue macrophage populations in controls. In the brain, microglial cell numbers and dendritic arborisation were reduced in Csf1rE631K/+ mice, as in patients with ALSP. The microglial phenotype is the opposite of microgliosis observed in Csf1r+/- mice. However, we found no evidence of brain pathology or impacts on motor function in aged Csf1rE631K/+ mice. We conclude that heterozygous disease-associated CSF1R mutations compromise CSF1R signalling. We speculate that leukoencephalopathy associated with dominant human CSF1R mutations requires an environmental trigger and/or epistatic interaction with common neurodegenerative disease-associated alleles.
Keywords: CSF1R; Kinase-dead; Leukoencephalopathy; Macrophage.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


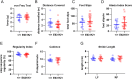
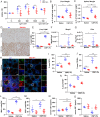
References
-
- Arreola, M. A., Soni, N., Crapser, J. D., Hohsfield, L. A., Elmore, M. R. P., Matheos, D. P., Wood, M. A., Swarup, V., Mortazavi, A. and Green, K. N. (2021). Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R(+/−) mouse model of ALSP, which can be rescued via CSF1R inhibitors. Sci. Adv. 7, eabg1601. 10.1126/sciadv.abg1601 - DOI - PMC - PubMed
-
- Bain, C. C., Hawley, C. A., Garner, H., Scott, C. L., Schridde, A., Steers, N. J., Mack, M., Joshi, A., Guilliams, M., Mowat, A. M.et al. (2016). Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun. 7, ncomms11852. 10.1038/ncomms11852 - DOI - PMC - PubMed
-
- Biundo, F., Chitu, V., Shlager, G. G. L., Park, E. S., Gulinello, M. E., Saha, K., Ketchum, H. C., Fernandes, C., Gökhan, Ş., Mehler, M. F.et al. (2020). Microglial reduction of colony stimulating factor-1 receptor expression is sufficient to confer adult onset leukodystrophy. Glia. 69:779-791. 10.1002/glia.23929 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

