Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss
- PMID: 35333444
- PMCID: PMC9310705
- DOI: 10.1002/oby.23374
Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss
Abstract
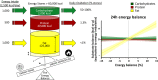
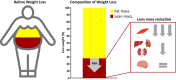
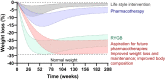
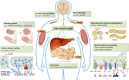
New appetite-regulating antiobesity treatments such as semaglutide and agents under investigation such as tirzepatide show promise in achieving weight loss of 15% or more. Energy expenditure, fat oxidation, and lean mass preservation are important determinants of weight loss and weight-loss maintenance beyond appetite regulation. This review discusses prior failures in clinical development of weight-loss drugs targeting energy expenditure and explores novel strategies for targeting energy expenditure: mitochondrial proton leak, uncoupling, dynamics, and biogenesis; futile calcium and substrate cycling; leptin for weight maintenance; increased sympathetic nervous system activity; and browning of white fat. Relevant targets for preserving lean mass are also reviewed: growth hormone, activin type II receptor inhibition, and urocortin 2 and 3. We endorse moderate modulation of energy expenditure and preservation of lean mass in combination with efficient appetite reduction as a means of obtaining a significant, safe, and long-lasting weight loss. Furthermore, we suggest that the regulatory guidelines should be revisited to focus more on the quality of weight loss and its maintenance rather than the absolute weight loss. Commitment to this research focus both from a scientific and from a regulatory point of view could signal the beginning of the next era in obesity therapies.
© 2022 The Authors. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society (TOS).
Conflict of interest statement
BØC, KR, and LMJ are full‐time employees and minor stockholders at Novo Nordisk A/S. GS‐D is a stockholder at BuenaVida Centro Integral de Salud. ER serves on the Scientific Advisory Board to the Nutrilite Health Institute with Amway and YSOPIA (LNC Therapeutics); has a consultant contract with Merck, Kintai Therapeutics, Big Sky Health, and Generian; and is an advisor for The Center for Medical Weight Loss. ER has received research grants or unrestricted gifts from Amway, Nestle, the Nutrition Science Initiative (NuSI), Weight Watchers, Lilly, Ethicon Surgery, Novartis, and Sanofi‐Avantis. Although he is Editor‐in‐Chief of
Figures

References
-
- Curry SJ, Krist AH, Owens DK, et al. Behavioral weight loss interventions to prevent obesity‐related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1163‐1171. - PubMed
-
- Enebo LB, Berthelsen KK, Kankam M, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397:1736‐1748. - PubMed
-
- Abbott WG, Howard BV, Christin L, et al. Short‐term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255:E332‐E337. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

