Examining the Impact of Tislelizumab Added to Chemotherapy on Health-Related Quality-of-Life Outcomes in Previously Untreated Patients With Nonsquamous Non-Small Cell Lung Cancer
- PMID: 35333492
- PMCID: PMC8974185
- DOI: 10.1097/PPO.0000000000000583
Examining the Impact of Tislelizumab Added to Chemotherapy on Health-Related Quality-of-Life Outcomes in Previously Untreated Patients With Nonsquamous Non-Small Cell Lung Cancer
Abstract
Purpose: This study assessed the effects of tislelizumab, a programmed cell death protein 1 inhibitor, in combination with chemotherapy versus chemotherapy alone as first-line treatment on health-related quality of life (HRQoL) in patients with advanced nonsquamous non-small cell lung cancer (nSQ-NSCLC).
Methods: Patients in this randomized, open-label, multicenter phase III study RATIONALE 304 (NCT03663205) with histologically confirmed stage IIIB/IV nSQ-NSCLC were randomized 2:1 to tislelizumab plus platinum-pemetrexed (arm T + PP) or platinum-pemetrexed alone (arm PP). Health-related QoL was measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Lung Cancer. Key patient-reported outcome endpoints include mean score change from baseline at weeks 12 (during chemotherapy) and 18 (following chemotherapy) in the 30-item Quality of Life Questionnaire Core's global health status/quality of life (GHS/QoL) and time to deterioration in GHS/QoL.
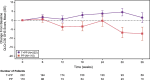
Results: Three hundred thirty-two patients received at least 1 dose of study drug and completed at least 1 HRQoL assessment. Global health status/QoL score improved in arm T + PP at week 18 (between-group least square mean difference, 5.7; 95% confidence interval [CI], 1.0-10.5; P = 0.018). Patients in arm T + PP experienced greater reduction in coughing (-5.9; 95% CI, -11.6 to -0.1; P = 0.044), dyspnea (-3.8; 95% CI, -7.8 to 0.1; P = 0.059), chest pain (-6.2; 95% CI, -10.8 to -1.6; P = 0.008), and peripheral neuropathy (-2.6; 95% CI, -5.5 to 0.2; P = 0.066). Median time to deterioration in GHS/QoL was not achieved for either arm.
Discussion: The addition of tislelizumab to platinum-based chemotherapy was associated with improvements in nSQ-NSCLC patients' HRQoL as well as the important disease-specific symptoms of coughing, chest pain, and dyspnea.ClinicalTrials.gov Identifier: NCT03663205.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest and Source of Funding: S.L. reports research support received from AstraZeneca, Hutchison, BMS, Heng Rui, and Roche; speaker fees received from AstraZeneca, Roche, BeiGene Ltd., and Hansoh; and advisor/consultant fees received from AstraZeneca, Boehringer Ingelheim, Hutchison MediPharma, Simcere, ZaiLab, GenomiCare, and Roche. G.B., X.Q., Y.B., and B.T. are employees of and own stock in BeiGene Ltd. For Y.Y., none were declared. This study was sponsored by BeiGene, Ltd. Support for the development of this manuscript was provided by BeiGene, Ltd.
Figures
References
-
- Bray F Ferlay J Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Chen W Zheng R Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Iyer S Roughley A Rider A, et al. The symptom burden of non–small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22:181–187. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

