High-Spin (S = 1) Blatter-Based Diradical with Robust Stability and Electrical Conductivity
- PMID: 35333507
- PMCID: PMC10439714
- DOI: 10.1021/jacs.2c01141
High-Spin (S = 1) Blatter-Based Diradical with Robust Stability and Electrical Conductivity
Abstract
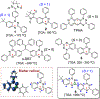
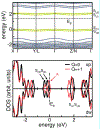
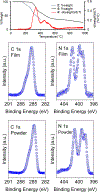
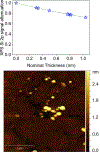
Triplet ground-state organic molecules are of interest with respect to several emerging technologies but usually show limited stability, especially as thin films. We report an organic diradical, consisting of two Blatter radicals, that possesses a triplet ground state with a singlet-triplet energy gap, ΔEST ≈ 0.4-0.5 kcal mol-1 (2J/k ≈ 220-275 K). The diradical possesses robust thermal stability, with an onset of decomposition above 264 °C (TGA). In toluene/chloroform, glassy matrix, and fluid solution, an equilibrium between two conformations with ΔEST ≈ 0.4 kcal mol-1 and ΔEST ≈ -0.7 kcal mol-1 is observed, favoring the triplet ground state over the singlet ground-state conformation in the 110-330 K temperature range. The diradical with the triplet ground-state conformation is found exclusively in crystals and in a polystyrene matrix. The crystalline neutral diradical is a good electrical conductor with conductivity comparable to the thoroughly optimized bis(thiazolyl)-related monoradicals. This is surprising because the triplet ground state implies that the underlying π-system is cross-conjugated and thus is not compatible with either good conductance or electron delocalization. The diradical is evaporated under ultra-high vacuum to form thin films, which are stable in air for at least 18 h, as demonstrated by X-ray photoelectron and electron paramagnetic resonance (EPR) spectroscopies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Gallagher NM; Bauer JJ; Pink M; Rajca S; Rajca A High-Spin Organic Diradical with Robust Stability. J. Am. Chem. Soc 2016, 138, 9377–9380. - PubMed
-
- Abe M Diradicals. Chem. Rev 2013, 113, 7011–7088. - PubMed
-
- Ratera I; Veciana J Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev 2012, 41, 303–349. - PubMed
-
- Junghoefer T; Gallagher N; Kolanji K; Giangrisostomi E; Ovsyannikov R; Chassé T; Baumgarten M; Rajca A; Calzolari A; Casu MB Challenges in controlled thermal deposition of organic diradicals. Chem. Mater 2021, 33, 2019–2028.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

