Structural determinants of dual incretin receptor agonism by tirzepatide
- PMID: 35333651
- PMCID: PMC9060465
- DOI: 10.1073/pnas.2116506119
Structural determinants of dual incretin receptor agonism by tirzepatide
Abstract
SignificanceTirzepatide is a dual agonist of the glucose-dependent insulinotropic polypeptide receptor (GIPR) and the glucagon-like peptide-1 receptor (GLP-1R), which are incretin receptors that regulate carbohydrate metabolism. This investigational agent has proven superior to selective GLP-1R agonists in clinical trials in subjects with type 2 diabetes mellitus. Intriguingly, although tirzepatide closely resembles native GIP in how it activates the GIPR, it differs markedly from GLP-1 in its activation of the GLP-1R, resulting in less agonist-induced receptor desensitization. We report how cryogenic electron microscopy and molecular dynamics simulations inform the structural basis for the unique pharmacology of tirzepatide. These studies reveal the extent to which fatty acid modification, combined with amino acid sequence, determines the mode of action of a multireceptor agonist.
Keywords: G protein coupled receptor (GPCR); GIP receptor; GLP-1 receptor; structure; tirzepatide.
Conflict of interest statement
Competing interest statement: F.S.W., J.A.-F., Q.C., M.V., J.D.H., A.D.S., C.S., L.D., T.M.S., J.D.D., J.W.C., F.A.M., E.A., R.A.B., A.B.B., P.J.E., J.S.M., M.P.C., and K.W.S. are employees of Eli Lilly and Company and may own company stock. B.S., D.F., T.S.K., and B.K.K. are employees of or consultants for ConfometRx. T.S.K. and B.K.K. cofounded ConfometRx.
Figures
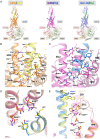
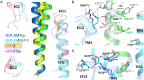

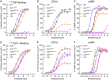

References
-
- Bokvist K. B., Coskun T., Cummins R. C., Alsina-Fernandez J., United States Patent No. US 9,474,780 B2. Available from: https://patentimages.storage.googleapis.com/e4/20/b1/04165a87d59f23/US94.... (2016).
-
- Samms R. J., Coghlan M. P., Sloop K. W., How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol. Metab. 31, 410–421 (2020). - PubMed
-
- Finan B., et al. , Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol. Med. 22, 359–376 (2016). - PubMed
-
- Frias J. P., et al. , Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

