Repeated cell sorting ensures the homogeneity of ocular cell populations expressing a transgenic protein
- PMID: 35333876
- PMCID: PMC8956163
- DOI: 10.1371/journal.pone.0265183
Repeated cell sorting ensures the homogeneity of ocular cell populations expressing a transgenic protein
Abstract
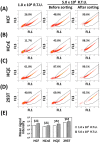
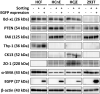
Transgenic proteins can be routinely expressed in various mammalian cell types via different transgenic systems, but the efficiency of transgene expression is constrained by the complex interplay among factors such as the temporal consistency of expression and compatibility with specific cell types, including ocular cells. Here, we report a more efficient way to express an enhanced green fluorescent protein (EGFP) in human corneal fibroblasts, corneal epithelial cells, and conjunctival epithelial cells through a lentiviral expression system. The relative transducing unit criterion for EGFP-expressing pseudovirions was first determined in HEK-293T cells. Homogeneous populations of EGFP-positive and EGFP-negative cells could be isolated by cell sorting. The half-maximal inhibitory concentration (IC50) value for puromycin was calculated according to viability curves for each cell type. The results revealed that cell types differed with respect to EGFP expression efficiency after transduction with the same amount of EGFP-encoding pseudovirions. Using a cell sorter, the homogeneity of EGFP-positive cells reached >95%. In the initial sorting stage, however, the efficiency of EGFP expression in the sorted cells was noticeably reduced after two rounds of sequential culture, but repeated sorting for up to four rounds yielded homogeneous EGFP-positive human corneal fibroblasts that could be maintained in continuous culture in vitro. The sorted EGFP-positive cells retained their proper morphology and cell type-specific protein expression patterns. Puromycin resistance was found to depend on cell type, indicating that the IC50 for puromycin must be determined for each cell type to ensure the isolation of homogeneous EGFP-positive cells. Taken together, repeated cell sorting is an efficient means of obtaining homogeneous populations of ocular cells expressing a transgenic protein during continuous culture without the potential confounding effects of antibiotics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Alipourfard I, Bakhtiyari S, Gheysarzadeh A, Di Renzo L, De Lorenzo A, Mikeladze D, et al. The key role of Akt protein kinase in metabolic-inflammatory pathways cross-talk: TNF-alpha down-regulation and improving of insulin resistance in HepG2 cell line. Curr Mol Med. 2020. Epub 2020/04/28. doi: 10.2174/1566524020666200427102209 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

