HIV-1 restriction by SERINC5
- PMID: 35333966
- PMCID: PMC10085909
- DOI: 10.1007/s00430-022-00732-x
HIV-1 restriction by SERINC5
Abstract
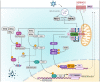
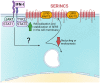
Serine incorporator 5 (SERINC5 or SER5) is a multipass transmembrane protein with ill-defined cellular activities. SER5 was recently described as a human immunodeficiency virus 1 (HIV-1) restriction factor capable of inhibiting HIV-1 that does not express its accessory protein Nef (Δ Nef). SER5 incorporated into the viral membrane impairs the entry of HIV-1 by disrupting the fusion between the viral and the plasma membrane after envelope receptor interaction induced the first steps of the fusion process. The mechanisms of how SER5 prevents membrane fusion are not fully understood and viral envelope proteins were identified that escape the SER5-mediated restriction. Primate lentiviruses, such as HIV-1 and simian immunodeficiency viruses (SIVs), use their accessory protein Nef to downregulate SER5 from the plasma membrane by inducing an endocytic pathway. In addition to being directly antiviral, recent data suggest that SER5 is an important adapter protein in innate signaling pathways leading to the induction of inflammatory cytokines. This review discusses the current knowledge about HIV-1 restriction by SER5.
Keywords: HIV-1; Nef; Restriction factors; SERINC5.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Editorial on special issue on "Immunobiology of Viral Infections".Med Microbiol Immunol. 2023 Apr;212(2):123-124. doi: 10.1007/s00430-023-00761-0. Med Microbiol Immunol. 2023. PMID: 36991263 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

