A multicentre, open-label, single-arm phase II trial of the efficacy and safety of sclerotherapy using 3% polidocanol foam to treat second-degree haemorrhoids (SCLEROFOAM)
- PMID: 35334004
- PMCID: PMC8949823
- DOI: 10.1007/s10151-022-02609-w
A multicentre, open-label, single-arm phase II trial of the efficacy and safety of sclerotherapy using 3% polidocanol foam to treat second-degree haemorrhoids (SCLEROFOAM)
Abstract
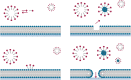
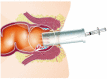
Background: The aim of the present study was to evaluate the efficacy and safety of 3% polidocanol foam for treating 2nd-degree haemorrhoids.
Methods: A multicentre, open-label, single-arm, phase 2 trial involving 10 tertiary referral centres for haemorrhodal disease (HD) was performed. Between January and June 2019, patients with 2nd-degree haemorrhoids were prospectively included in this study. The primary outcome was to establish the success rate after one sclerotherapy session in terms of complete resolution of bleeding episodes one week after the injection. The Hemorrhoidal Disease Symptom Score (HDSS), the Short Health Scale for HD (SHS-HD) score and the Vaizey incontinence score were used to assess symptoms and their impact on quality of life and continence. Pain after the procedure, subjective symptoms and the amount and type of painkillers used were recorded. Patients were followed up for 1 year.
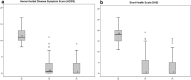
Results: There were 183 patients [111 males; 60.7%, mean age 51.3 ± 13.5 (18-75) years]. Complete resolution of bleeding was reached in 125/183 patients (68.3%) at 1 week and the recurrence rate was 12% (15/125). Thirteen patients (7.4%) underwent a second sclerotherapy session, while only 1 patient (1.8%) had to undergo a third session. The overall 1-year success rate was 95.6% (175/183). The HDSS and the SHS score significantly improved from a median preoperative value of 11 and 18 to 0 and 0, respectively (p < 0.001). There were 3 episodes of external thrombosis. No serious adverse events occurred.
Conclusions: Sclerotherapy with 3% polidocanol foam is a safe, effective, painless, repeatable and low-cost procedure in patients with bleeding haemorrhoids.
Keywords: Bleeding haemorrhoids; Haemorrhoidal disease; Polidocanol foam; Safety; Sclerotherapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
The comeback of hemorrhoidal sclerotherapy?Tech Coloproctol. 2022 Aug;26(8):599-601. doi: 10.1007/s10151-022-02640-x. Tech Coloproctol. 2022. PMID: 35737212 No abstract available.
References
-
- Gallo G, Sacco R, Sammarco G. Epidemiology of hemorrhoidal disease. In: Ratto C, Parello A, Litta F, editors. Hemorrhoids Coloproctology. Cham: Springer; 2018. pp. 3–7.
-
- Gallo G, Martellucci J, Sturiale A, Clerico G, Milito G, Marino F, Cocorullo G, Giordano P, Mistrangelo M, Trompetto M. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol. 2020;24(2):145–164. doi: 10.1007/s10151-020-02149-1. - DOI - PMC - PubMed
-
- Elbetti C, Giani I, Novelli E, Martellucci J, Feroci F. Symptomatic pile tailored procedure. A new perspective for hemorrhoidal disease treatment. Ann Ital Chir. 2017;88:348–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

