NAD+ centric mechanisms and molecular determinants of skeletal muscle disease and aging
- PMID: 35334034
- PMCID: PMC10065019
- DOI: 10.1007/s11010-022-04408-1
NAD+ centric mechanisms and molecular determinants of skeletal muscle disease and aging
Abstract
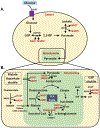
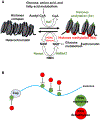
The nicotinamide adenine dinucleotide (NAD+) is an essential redox cofactor, involved in various physiological and molecular processes, including energy metabolism, epigenetics, aging, and metabolic diseases. NAD+ repletion ameliorates muscular dystrophy and improves the mitochondrial and muscle stem cell function and thereby increase lifespan in mice. Accordingly, NAD+ is considered as an anti-oxidant and anti-aging molecule. NAD+ plays a central role in energy metabolism and the energy produced is used for movements, thermoregulation, and defense against foreign bodies. The dietary precursors of NAD+ synthesis is targeted to improve NAD+ biosynthesis; however, studies have revealed conflicting results regarding skeletal muscle-specific effects. Recent advances in the activation of nicotinamide phosphoribosyltransferase in the NAD+ salvage pathway and supplementation of NAD+ precursors have led to beneficial effects in skeletal muscle pathophysiology and function during aging and associated metabolic diseases. NAD+ is also involved in the epigenetic regulation and post-translational modifications of proteins that are involved in various cellular processes to maintain tissue homeostasis. This review provides detailed insights into the roles of NAD+ along with molecular mechanisms during aging and disease conditions, such as the impacts of age-related NAD+ deficiencies on NAD+-dependent enzymes, including poly (ADP-ribose) polymerase (PARPs), CD38, and sirtuins within skeletal muscle, and the most recent studies on the potential of nutritional supplementation and distinct modes of exercise to replenish the NAD+ pool.
Keywords: Aging; Diabetes; Epigenetics; Muscle diseases; NAD+; Redox.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of interest
All authors declared that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

