Immunoglobulin G1 Fc glycosylation as an early hallmark of severe COVID-19
- PMID: 35334306
- PMCID: PMC8938159
- DOI: 10.1016/j.ebiom.2022.103957
Immunoglobulin G1 Fc glycosylation as an early hallmark of severe COVID-19
Abstract
Background: Immunoglobulin G1 (IgG1) effector functions are impacted by the structure of fragment crystallizable (Fc) tail-linked N-glycans. Low fucosylation levels on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein-specific IgG1 has been described as a hallmark of severe coronavirus disease 2019 (COVID-19) and may lead to activation of macrophages via immune complexes thereby promoting inflammatory responses, altogether suggesting involvement of IgG1 Fc glycosylation modulated immune mechanisms in COVID-19.
Methods: In this prospective, observational single center cohort study, IgG1 Fc glycosylation was analyzed by liquid chromatography-mass spectrometry following affinity capturing from serial plasma samples of 159 SARS-CoV-2 infected hospitalized patients.
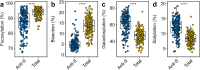
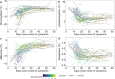
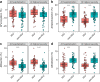
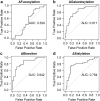
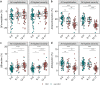
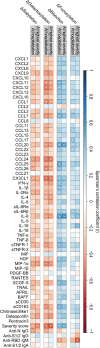
Findings: At baseline close to disease onset, anti-S IgG1 glycosylation was highly skewed when compared to total plasma IgG1. A rapid, general reduction in glycosylation skewing was observed during the disease course. Low anti-S IgG1 galactosylation and sialylation as well as high bisection were early hallmarks of disease severity, whilst high galactosylation and sialylation and low bisection were found in patients with low disease severity. In line with these observations, anti-S IgG1 glycosylation correlated with various inflammatory markers.
Interpretation: Association of low galactosylation, sialylation as well as high bisection with disease severity and inflammatory markers suggests that further studies are needed to understand how anti-S IgG1 glycosylation may contribute to disease mechanism and to evaluate its biomarker potential.
Funding: This project received funding from the European Commission's Horizon2020 research and innovation program for H2020-MSCA-ITN IMforFUTURE, under grant agreement number 721815, and supported by Crowdfunding Wake Up To Corona, organized by the Leiden University Fund.
Keywords: Anti-spike IgG; COVID-19; Coronavirus; IgG glycosylation; SARS-CoV-2.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests A. H. E. R received support from Crowdfunding Wake Up To Corona, organized by the Leiden University Fund, participated in grants or contracts with Diorapthe, Stichting apothekers and UNeedle, participated on a Data Safety Monitoring/Advisory Board of a multicenter Dutch clinical trial (Clinical trial (RCT) on convalescent plasma for treatment of immunocompromised patients with COVID-19) and has recently been appointed as member of the EMA scientific advisory group on vaccines (unpaid). The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Long Q.X., Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

