Sodium thiosulfate acts as a hydrogen sulfide mimetic to prevent intimal hyperplasia via inhibition of tubulin polymerisation
- PMID: 35334307
- PMCID: PMC8941337
- DOI: 10.1016/j.ebiom.2022.103954
Sodium thiosulfate acts as a hydrogen sulfide mimetic to prevent intimal hyperplasia via inhibition of tubulin polymerisation
Abstract
Background: Intimal hyperplasia (IH) remains a major limitation in the long-term success of any type of revascularisation. IH is due to vascular smooth muscle cell (VSMC) dedifferentiation, proliferation and migration. The gasotransmitter Hydrogen Sulfide (H2S), mainly produced in blood vessels by the enzyme cystathionine- γ-lyase (CSE), inhibits IH in pre-clinical models. However, there is currently no H2S donor available to treat patients. Here we used sodium thiosulfate (STS), a clinically-approved source of sulfur, to limit IH.
Methods: Low density lipoprotein receptor deleted (LDLR-/-), WT or Cse-deleted (Cse-/-) male mice randomly treated with 4 g/L STS in the water bottle were submitted to focal carotid artery stenosis to induce IH. Human vein segments were maintained in culture for 7 days to induce IH. Further in vitro studies were conducted in primary human vascular smooth muscle cells (VSMCs).
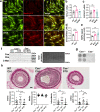
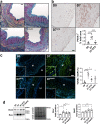
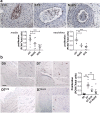
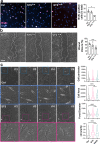
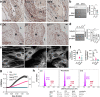
Findings: STS inhibited IH in WT mice, as well as in LDLR-/- and Cse-/- mice, and in human vein segments. STS inhibited cell proliferation in the carotid artery wall and in human vein segments. STS increased polysulfides in vivo and protein persulfidation in vitro, which correlated with microtubule depolymerisation, cell cycle arrest and reduced VSMC migration and proliferation.
Interpretation: STS, a drug used for the treatment of cyanide poisoning and calciphylaxis, protects against IH in a mouse model of arterial restenosis and in human vein segments. STS acts as an H2S donor to limit VSMC migration and proliferation via microtubule depolymerisation.
Funding: This work was supported by the Swiss National Science Foundation (grant FN-310030_176158 to FA and SD and PZ00P3-185927 to AL); the Novartis Foundation to FA; and the Union des Sociétés Suisses des Maladies Vasculaires to SD, and the Fondation pour la recherche en chirurgie vasculaire et thoracique.
Keywords: Hydrogen sulfide; Intimal hyperplasia; Proliferation; Smooth muscle cells; Sodium thiosulfate.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Fowkes F.G., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. - PubMed
-
- Song P., Rudan D., Zhu Y., et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7(8):e1020–e1030. - PubMed
-
- Bjorck M., Earnshaw J.J., Acosta S., et al. Editor's choice - European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59(2):173–218. - PubMed
-
- Jukema J.W., Verschuren J.J., Ahmed T.A., Quax P.H. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9(1):53–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

