Interactions between glucosides of the tip of the S1 subunit of SARS-CoV-2 spike protein and dry and wet surfaces of CuO and Cu-A model for the surfaces of coinage metals
- PMID: 35334309
- PMCID: PMC8940556
- DOI: 10.1016/j.colsurfb.2022.112465
Interactions between glucosides of the tip of the S1 subunit of SARS-CoV-2 spike protein and dry and wet surfaces of CuO and Cu-A model for the surfaces of coinage metals
Abstract
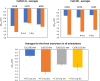
Despite their importance there is little knowledge at the atomic scale on the interactions between fragments of SARS-CoV-2 and inorganic materials. Such knowledge is important to understand the survival of the virus at surfaces and for the development of antiviral materials. Here is reported a study of the interactions between glucoside monomers of the tip of the S1 subunit of SARS-CoV-2 spike protein with dry and wet surfaces of CuO and Cu, performed with dispersion corrected density functional theory-DFT. The three glucoside monomers that constitute the tip of S1: 6VSB, 6VXX and 6X6P, were adsorbed onto dry and wet CuO(111) and Cu(110) with different orientations and surface alignments. There are large differences-of up to 1.3 eV-in binding energies between these monomers and the surfaces. These differences depend on: the type of surface; if the surface is wet or dry; if the glucosidic O-atom points towards or away from the surfaces; and to a smaller extent on the surface alignment of the monomers. All monomers bind strongly to the surfaces via molecular adsorption that does not involve bond breaking in the monomers at this stage. 6VSB has the larger adsorption energies-that reach 2.2 eV-due to its larger dipole moment. Both materials bind the monomers more strongly when their surfaces are dry. At Cu(110) the bonds are on average 1 eV stronger when the surface is dry when compared to wet. The difference between dry and wet CuO(111) is smaller, in the order of 0.2 eV. Overall, it is here shown that the stability of the monomers of the tip of the spike protein of the virus is very different at different surfaces. For a given surface the larger binding energies in dry conditions could explain the differences in the surface stability of the spike protein depending on the presence of moisture.
Keywords: DFT; Interactions with inorganic surfaces; Quantum mechanical modeling; SARS-CoV-2; Spike protein.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

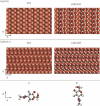
 ), O (
), O ( ), H (○).
), H (○).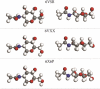
 ), O (
), O ( ), N (
), N ( ) H (○). The conformation 6VSB is β while 6VXX and 6X6P are α.
) H (○). The conformation 6VSB is β while 6VXX and 6X6P are α.
Similar articles
-
Computer simulations of the interaction between SARS-CoV-2 spike glycoprotein and different surfaces.Biointerphases. 2020 Oct 26;15(5):051008. doi: 10.1116/6.0000502. Biointerphases. 2020. PMID: 33105999
-
Computer Simulation of the Interaction between SARS-CoV-2 Spike Protein and the Surface of Coinage Metals.Langmuir. 2022 Dec 6;38(48):14673-14685. doi: 10.1021/acs.langmuir.2c02120. Epub 2022 Nov 23. Langmuir. 2022. PMID: 36418228 Free PMC article.
-
Static all-atom energetic mappings of the SARS-Cov-2 spike protein and dynamic stability analysis of "Up" versus "Down" protomer states.PLoS One. 2020 Nov 10;15(11):e0241168. doi: 10.1371/journal.pone.0241168. eCollection 2020. PLoS One. 2020. PMID: 33170884 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
References
-
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New Engl. J. Med. 2020;382:1564–1567. - PMC - PubMed
-
- Luntz A.C. In: Chemical Bonding at Surfaces and Interfaces. Nilsson A., et al., editors. Elsevier; Amsterdam: 2008. pp. 143–254.
-
- Nilsson A., Pettersson L.G.M. In: Chemical Bonding at Surfaces and Interfaces. Nilsson A., et al., editors. Elsevier; Amsterdam: 2008. pp. 57–142.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

