The Anti-Endometriotic Effect of Cyperi Rhizoma Extract, Inhibiting Cell Adhesion and the Expression of Pain-Related Factors through Akt and NF-kB Pathways
- PMID: 35334511
- PMCID: PMC8953559
- DOI: 10.3390/medicina58030335
The Anti-Endometriotic Effect of Cyperi Rhizoma Extract, Inhibiting Cell Adhesion and the Expression of Pain-Related Factors through Akt and NF-kB Pathways
Abstract
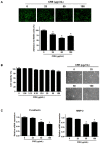
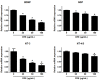
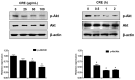
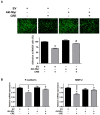
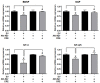
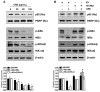
Rhizomes of Cyperus rotundus have been widely used as a traditional medicine in Asia for the treatment of gynecological diseases. However, there is no scientific evidence demonstrating the effect of C. rotundus rhizomes on endometriosis, which is characterized by the adhesion of endometrial tissues outside the uterus, resulting in chronic and severe pelvic pain. The aim of this study was to investigate the effects of Cyperi rhizoma extract (CRE) on cell adhesion and the expression of pain-related factors (neurotrophins) in endometriotic cells, and to elucidate the underlying molecular mechanisms. CRE inhibited the adhesion of human endometriotic 12Z cells to peritoneal mesothelial Met5A cells using by adhesion assays. The mRNA expression of adhesion molecules [P-cadherin and matrix metalloproteinase (MMP)-2] was downregulated by CRE treatment. In addition, CRE significantly inhibited the mRNA expression of neurotrophins (BDNF, NGF, NT-3 and NT-4/5) in 12Z cells. Moreover, Akt overexpression markedly neutralized the inhibition of cell adhesion by CRE and expression of neurotrophins in 12Z cells. Furthermore, it was found that CRE suppressed NF-kB activation through the Akt pathway. These data suggest that CRE exerts anti-endometriotic activities by the inhibition of cell adhesion and neurotrophin expression, through the negative regulation of the Akt and NF-kB pathways in endometriotic cells.
Keywords: Akt; Cyperi rhizome; NF-kB; adhesion; endometriosis; neurotrophins.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

