A Pathological Complete Response to the Combination of Ipilimumab and Nivolumab in a Patient with Metastatic Renal Cell Carcinoma
- PMID: 35334512
- PMCID: PMC8951627
- DOI: 10.3390/medicina58030336
A Pathological Complete Response to the Combination of Ipilimumab and Nivolumab in a Patient with Metastatic Renal Cell Carcinoma
Abstract
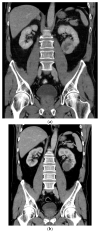
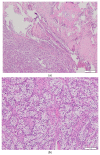
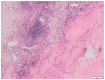
Background and Objectives: Complete pathological response after ipilimumab and nivolumab combination therapy in a patient with intermediate prognosis renal cell carcinoma is an uncommon finding. Case presentation: A 60-year-old man presented with synchronous solitary metastatic bone lesion and renal cell carcinoma and achieved a complete pathological response after surgical resection of the bone lesion, followed by ipilimumab and nivolumab combination therapy and nephrectomy. The treatment was complicated by hypophysitis and oligoarthritis more than a year after the initiation of the therapy. Conclusions: Currently, the combination therapy based on immune checkpoint inhibitors represents the treatment of choice in patients with intermediate- and poor-risk prognosis metastatic renal cell carcinoma. In the present case, preoperative therapy with ipilimumab and nivolumab resulted in a complete pathological response in the renal tumor. Vigilance concerning potential immune-related side effects is warranted throughout the course of therapy and the subsequent follow-up.
Keywords: hypophysitis; immunotherapy; pathological complete response; renal cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., Gerber B., Eiermann W., Hilfrich J., Huober J., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. - DOI - PubMed
-
- Chollet P., Amat S., Cure H., de Latour M., Le Bouedec G., Mouret-Reynier M.A., Ferreiere J.P., Achard J.L., Dauplat J., Penault-Llorca F. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br. J. Cancer. 2002;86:1041–1046. doi: 10.1038/sj.bjc.6600210. - DOI - PMC - PubMed
-
- Bear H.D., Anderson S., Smith R.E., Geyer C.E., Mamounas E.P., Fisher B., Brown A.M., Robidoux A., Margolese R., Kahlenberg M.S., et al. Sequential Preoperative or Postoperative Docetaxel Added to Preoperative Doxorubicin Plus Cyclophosphamide for Operable Breast Cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. - DOI - PubMed
-
- Machiavelli M.R., Romero A.O., Pérez J.E., Lacava J.A., Domínguez M.E., Rodríguez R., Barbieri M.R., Acuña L.A.R., Acuña J.M.R., Langhi M.J., et al. Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J. Sci. Am. 1998;4:125–131. - PubMed
-
- Vitásková D., Melichar B., Bartoušková M., Vlachová Z., Vrána D., Janková J., Adam T., Juráňová J., Zlámalová N., Krčmová L.K., et al. Neoadjuvant combination therapy with trastuzumab in a breast cancer patient with synchronous rectal carcinoma: A case report and biomarker study. Pteridines. 2017;28:233–241. doi: 10.1515/pterid-2017-0017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

