Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice
- PMID: 35334805
- PMCID: PMC8951758
- DOI: 10.3390/nu14061148
Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice
Abstract
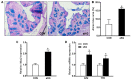
The metabolite, alpha-ketoglutarate (aKG), shows promise as an approach for ameliorating colitis, but much remains unknown about the full extent of its effects on the metabolome and mucosal barrier. To further elucidate this matter, C57BL/6 male mice received drinking water with or without 1% aKG for three weeks, then were subjected to 2.5% dextran sulfate sodium (DSS) induction for 7 days followed by 7 days of recovery. Cecal content and intestinal tissue samples were analyzed for changes in metabolite profile and signaling pathways. Gas chromatography-mass spectrometry (GC-MS) metabolomics revealed a separation between the metabolome of mice treated with or without aKG; putrescine and glycine were significantly increased; and ornithine and amide products, oleamide and urea were significantly decreased. Based on a pathway analysis, aKG treatment induced metabolite changes and enriched glutathione metabolism and the urea cycle. Additionally, signaling pathways committing epithelial cells to the secretory lineage were elevated in aKG-treated mice. Consistently, aKG supplementation increased goblet cells staining, mRNA expression of mucin 2, and, trefoil factor 3 and Krüppel-like factor 4, markers of goblet cell differentiation. These data suggest the ameliorating the effects of aKG against chemically induced colitis involves a reduction in harmful metabolites and the promotion of goblet cell differentiation, resulting in a more-fortified mucus layer.
Keywords: alpha-ketoglutarate; colitis; goblet cells; metabolome; mucin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



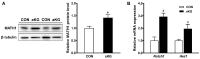
References
-
- Khaloian S., Rath E., Hammoudi N., Gleisinger E., Blutke A., Giesbertz P., Berger E., Metwaly A., Waldschmitt N., Allez M., et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut. 2020;69:1939–1951. doi: 10.1136/gutjnl-2019-319514. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

